Biomaterials ( IF 12.8 ) Pub Date : 2020-06-10 , DOI: 10.1016/j.biomaterials.2020.120181 Chao He 1 , Luodan Yu 2 , Li Ding 2 , Heliang Yao 3 , Yu Chen 2 , Yongqiang Hao 1
|
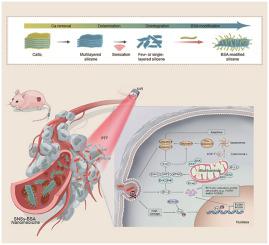
Breast cancer (BC) is the most common malignant disease affecting women's health worldwide. The benefits from conventional therapeutic modalities are severely limited. An increasing number of promising photothermal materials have been recently developed and introduced into the therapeutic regimens of BC, but the underlying biological mechanism remains unclear. Silicon-based materials have enjoyed many popularities in the biomedical field owing to their desirable biocompatibility, biodegradability and versatility. Herein, we introduced two dimensional (2D) silicene nanosheets (SNSs) into the BC treatment and achieved profound photothermal-ablation efficacy. Importantly, this work reveals the underlying biological mechanism and regulation factors of photonic hyperthermia in BC. The RNA sequencing and immunoblot demonstrated that photothermia enhanced apoptosis in BC by activating caspase 3 and caspase 7. Importantly, knockdown of lysine demethylase KDM3A sensitized BC to photothermia epigenetically. It has been revealed that KDM3A could erase p53K372me1 and suppress the anti-cancer functions of p53, leading to the downregulation of pro-apoptotic proteins-PUMA and NOXA verified by Co-IP and ChIP-qPCR assays. Therefore, our results not only import near infrared light (NIR) induced photothermal ablation generated by SNSs-BSA into the BC treatment, but also clarify the underlying mechanism and regulation factors for further photothermal performance optimization and clinical translation.
中文翻译:

赖氨酸去甲基化酶 KDM3A 调节乳腺癌中二维硅烯产生的纳米光子热疗抗性。
乳腺癌(BC)是影响全球女性健康的最常见的恶性疾病。传统治疗方式的益处受到严重限制。最近开发了越来越多的有前途的光热材料并将其引入 BC 的治疗方案,但潜在的生物学机制仍不清楚。硅基材料因其理想的生物相容性、生物降解性和多功能性而在生物医学领域享有盛誉。在此,我们将二维 (2D) 硅烯纳米片 (SNS) 引入到 BC 处理中,并实现了显着的光热消融效果。重要的是,这项工作揭示了 BC 光子热疗的潜在生物学机制和调节因素。RNA 测序和免疫印迹表明,光热通过激活半胱天冬酶 3 和半胱天冬酶 7 增强了 BC 的细胞凋亡。重要的是,赖氨酸去甲基化酶 KDM3A 的敲低使 BC 在表观遗传上对光热敏感。已经发现 KDM3A 可以擦除 p53K372me1 并抑制 p53 的抗癌功能,导致通过 Co-IP 和 ChIP-qPCR 测定验证的促凋亡蛋白-PUMA 和 NOXA 的下调。因此,我们的研究结果不仅将 SNSs-BSA 产生的近红外光 (NIR) 诱导光热消融引入 BC 治疗,而且阐明了进一步光热性能优化和临床转化的潜在机制和调控因素。赖氨酸去甲基化酶 KDM3A 的敲低使 BC 在表观遗传上对光热敏感。已经发现 KDM3A 可以擦除 p53K372me1 并抑制 p53 的抗癌功能,导致通过 Co-IP 和 ChIP-qPCR 测定验证的促凋亡蛋白-PUMA 和 NOXA 的下调。因此,我们的研究结果不仅将 SNSs-BSA 产生的近红外光 (NIR) 诱导光热消融引入 BC 治疗,而且阐明了进一步光热性能优化和临床转化的潜在机制和调控因素。赖氨酸去甲基化酶 KDM3A 的敲低使 BC 在表观遗传上对光热敏感。已经发现 KDM3A 可以擦除 p53K372me1 并抑制 p53 的抗癌功能,导致通过 Co-IP 和 ChIP-qPCR 测定验证的促凋亡蛋白-PUMA 和 NOXA 的下调。因此,我们的研究结果不仅将 SNSs-BSA 产生的近红外光 (NIR) 诱导光热消融引入 BC 治疗,而且阐明了进一步光热性能优化和临床转化的潜在机制和调控因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号