当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fragmentation Patterns of Radiosensitizers Metronidazole and Nimorazole upon Valence Ionization.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jpca.0c03045 Eero Itälä 1 , Johannes Niskanen 1 , Lassi Pihlava 1 , Edwin Kukk 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jpca.0c03045 Eero Itälä 1 , Johannes Niskanen 1 , Lassi Pihlava 1 , Edwin Kukk 1
Affiliation
|
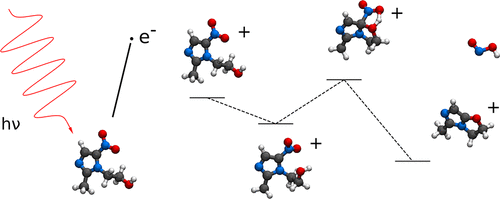
We study gas-phase photodissociation of radiosensitizer molecules nimorazole and metronidazole with the focus on the yield of the oxygen mimics nitrogen oxides and nitrous acid. Regardless of photon energy, we find the nimorazole cation to split the intramolecular bridge with little NO2 or NO production, which makes the molecule a precursor of dehydrogenated methylnitroimidazole. Metronidazole cation, on the contrary, has numerous fragmentation pathways with strong energy dependence. Most notably, ejection of NOOH and NO2 takes place within 4 eV from the valence ionization energy. Whereas the NO2 ejection is followed by further fragmentation steps when energy so allows, we find emission of NOOH takes place in microsecond time-scales and as a slow process that is relevant only when no other competing reaction is feasible. These primary dissociation characteristics of the molecules are understood by applying the long-known principle of rapid internal conversion of the initial electronic excitation energy and by studying the energy minima and the saddle points on the potential energy surface of the electronic ground state of the molecular cation.
中文翻译:

价电离后,放射增敏剂甲硝唑和尼莫拉唑的裂解模式。
我们研究放射增敏剂分子尼莫拉唑和甲硝唑的气相光解离,重点研究模拟氮氧化物和亚硝酸的氧收率。无论光子能量如何,我们都发现尼莫拉唑阳离子可以分裂分子内桥,几乎没有NO 2或NO生成,这使该分子成为脱氢甲基硝基咪唑的前体。相反,甲硝唑阳离子具有许多具有强烈能量依赖性的裂解途径。最值得注意的是,NOOH和NO 2的排放在离化合价电离能4 eV的范围内发生。而NO 2在能量允许的情况下,喷射之后是进一步的破碎步骤,我们发现NOOH的排放发生在微秒级的时间范围内,并且是一个缓慢的过程,仅在没有其他竞争性反应可行时才相关。通过应用众所周知的初始电子激发能的快速内部转换原理,以及通过研究分子阳离子的电子基态势能表面上的最小能量和鞍点,可以了解分子的这些主要解离特征。
更新日期:2020-07-09
中文翻译:

价电离后,放射增敏剂甲硝唑和尼莫拉唑的裂解模式。
我们研究放射增敏剂分子尼莫拉唑和甲硝唑的气相光解离,重点研究模拟氮氧化物和亚硝酸的氧收率。无论光子能量如何,我们都发现尼莫拉唑阳离子可以分裂分子内桥,几乎没有NO 2或NO生成,这使该分子成为脱氢甲基硝基咪唑的前体。相反,甲硝唑阳离子具有许多具有强烈能量依赖性的裂解途径。最值得注意的是,NOOH和NO 2的排放在离化合价电离能4 eV的范围内发生。而NO 2在能量允许的情况下,喷射之后是进一步的破碎步骤,我们发现NOOH的排放发生在微秒级的时间范围内,并且是一个缓慢的过程,仅在没有其他竞争性反应可行时才相关。通过应用众所周知的初始电子激发能的快速内部转换原理,以及通过研究分子阳离子的电子基态势能表面上的最小能量和鞍点,可以了解分子的这些主要解离特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号