当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A peroxodiiron(III) intermediate mediating both N-hydroxylation steps in biosynthesis of the N-nitrosourea pharmacophore of streptozotocin by SznF
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-08 , DOI: 10.1021/jacs.0c03431 Molly J McBride , Debangsu Sil , Tai L Ng 1 , Anne Marie Crooke 1 , Grace E Kenney 1 , Christina R Tysoe , Bo Zhang , Emily P Balskus 1 , Amie K Boal , Carsten Krebs , J Martin Bollinger
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-08 , DOI: 10.1021/jacs.0c03431 Molly J McBride , Debangsu Sil , Tai L Ng 1 , Anne Marie Crooke 1 , Grace E Kenney 1 , Christina R Tysoe , Bo Zhang , Emily P Balskus 1 , Amie K Boal , Carsten Krebs , J Martin Bollinger
Affiliation
|
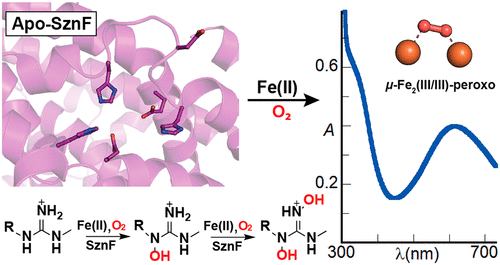
The alkylating warhead of the antineoplastic drug streptozotocin (SZN) contains an N-nitrosourea moiety constructed from Nω-methyl-L-arginine (L-NMA) by the multi-domain metalloenzyme SznF. The enzyme's central heme-oxygenase-like (HO-like) domain sequentially hydroxylates Nδ and Nω' of L-NMA. Its C-terminal cupin domain then rearranges the triply modified arginine to Nδ-hydroxy-Nω-methyl-Nω-nitroso-L-citrulline, the proposed donor of the functional pharmacophore. Here we show that the HO-like domain of SznF can bind Fe(II) and use it to capture O2, forming a peroxo-Fe2(III/III) intermediate. This intermediate has absorption- and Mössbauer-spectroscopic features similar to those of complexes previously trapped in ferritin-like diiron oxidases and oxygenases (FDOs) and, more recently, the HO-like fatty acid oxidase UndA. The SznF peroxo-Fe2(III/III) complex is an intermediate in both hydroxylation steps, as shown by the concentration-dependent acceleration of its decay upon exposure to either L-NMA or Nδ-hydroxy-Nω-methyl-L-Arg (L-HMA). The Fe2(III/III) cluster produced upon decay of the intermediate has a small Mössbauer quadrupole splitting parameter, implying that, unlike the corresponding product states of many FDOs, it lacks an oxo-bridge. The subsequent decomposition of the product cluster to one or more paramagnetic Fe(III) species over several hours explains why SznF was previously purified and crystallographically characterized without its cofactor. Programmed instability of the oxidized form of the cofactor appears to be a unifying characteristic of the emerging superfamily of HO-like diiron oxidases and oxygenases (HDOs).
中文翻译:

过氧化二铁 (III) 中间体介导 SznF 链脲佐菌素 N-亚硝基脲药效团生物合成中的两个 N-羟基化步骤
抗肿瘤药物链脲佐菌素 (SZN) 的烷基化弹头含有一个 N-亚硝基脲部分,该部分由多域金属酶 SznF 由 Nω-甲基-L-精氨酸 (L-NMA) 构建而成。该酶的中心血红素加氧酶样(HO 样)结构域依次羟基化 L-NMA 的 Nδ 和 Nω'。然后,其 C 端 cupin 结构域将三重修饰的精氨酸重新排列为 Nδ-羟基-Nω-甲基-Nω-亚硝基-L-瓜氨酸,即功能药效团的拟议供体。在这里,我们证明 SznF 的 HO 样结构域可以结合 Fe(II) 并用它来捕获 O2,形成过氧-Fe2(III/III) 中间体。这种中间体具有与先前被困在铁蛋白样二铁氧化酶和加氧酶 (FDO) 以及最近的 H2O 样脂肪酸氧化酶 UndA 中的复合物类似的吸收光谱和穆斯堡尔光谱特征。 SznF peroxo-Fe2(III/III) 络合物是两个羟基化步骤中的中间体,如暴露于 L-NMA 或 Nδ-羟基-Nω-甲基-L-Arg 时其衰变的浓度依赖性加速所示。 L-HMA)。中间体衰变时产生的 Fe2(III/III) 簇具有较小的穆斯堡尔四极分裂参数,这意味着与许多 FDO 的相应产物态不同,它缺乏氧桥。随后在几个小时内产物簇分解为一种或多种顺磁性 Fe(III) 物质,这解释了为什么先前纯化 SznF 并在没有其辅因子的情况下进行晶体学表征。辅因子氧化形式的程序化不稳定性似乎是新兴的 H2O 样二铁氧化酶和加氧酶 (HDO) 超家族的统一特征。
更新日期:2020-06-08
中文翻译:

过氧化二铁 (III) 中间体介导 SznF 链脲佐菌素 N-亚硝基脲药效团生物合成中的两个 N-羟基化步骤
抗肿瘤药物链脲佐菌素 (SZN) 的烷基化弹头含有一个 N-亚硝基脲部分,该部分由多域金属酶 SznF 由 Nω-甲基-L-精氨酸 (L-NMA) 构建而成。该酶的中心血红素加氧酶样(HO 样)结构域依次羟基化 L-NMA 的 Nδ 和 Nω'。然后,其 C 端 cupin 结构域将三重修饰的精氨酸重新排列为 Nδ-羟基-Nω-甲基-Nω-亚硝基-L-瓜氨酸,即功能药效团的拟议供体。在这里,我们证明 SznF 的 HO 样结构域可以结合 Fe(II) 并用它来捕获 O2,形成过氧-Fe2(III/III) 中间体。这种中间体具有与先前被困在铁蛋白样二铁氧化酶和加氧酶 (FDO) 以及最近的 H2O 样脂肪酸氧化酶 UndA 中的复合物类似的吸收光谱和穆斯堡尔光谱特征。 SznF peroxo-Fe2(III/III) 络合物是两个羟基化步骤中的中间体,如暴露于 L-NMA 或 Nδ-羟基-Nω-甲基-L-Arg 时其衰变的浓度依赖性加速所示。 L-HMA)。中间体衰变时产生的 Fe2(III/III) 簇具有较小的穆斯堡尔四极分裂参数,这意味着与许多 FDO 的相应产物态不同,它缺乏氧桥。随后在几个小时内产物簇分解为一种或多种顺磁性 Fe(III) 物质,这解释了为什么先前纯化 SznF 并在没有其辅因子的情况下进行晶体学表征。辅因子氧化形式的程序化不稳定性似乎是新兴的 H2O 样二铁氧化酶和加氧酶 (HDO) 超家族的统一特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号