当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Effect of Sterically Active Ligand Substituents on Gas Adsorption within a Family of 3D Zn-Based Coordination Polymers.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.inorgchem.0c00684 Brendan F Abrahams 1 , Ravichandar Babarao 2, 3 , A David Dharma 1 , Jessica L Holmes 1 , Timothy A Hudson 1 , Helen E Maynard-Casely 4 , Forbes McGain 1, 5 , Richard Robson 1 , Keith F White 1, 6
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.inorgchem.0c00684 Brendan F Abrahams 1 , Ravichandar Babarao 2, 3 , A David Dharma 1 , Jessica L Holmes 1 , Timothy A Hudson 1 , Helen E Maynard-Casely 4 , Forbes McGain 1, 5 , Richard Robson 1 , Keith F White 1, 6
Affiliation
|
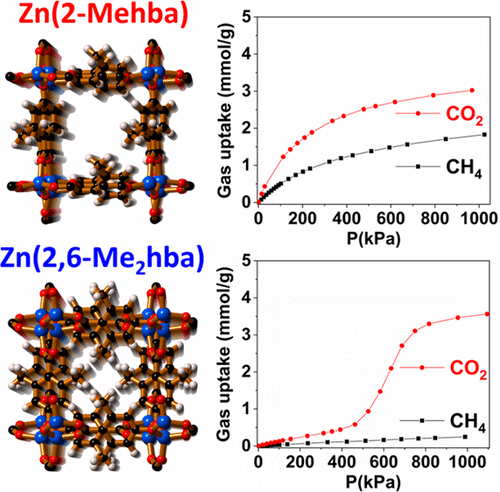
An investigation of the adsorption properties of two structurally related, 3D coordination polymers of composition Zn(2-Mehba) and Zn(2,6-Me2hba) (2-Mehba = the dianion of 2-methyl-4-hydroxybenzoic acid and 2,6-Me2hba = the dianion of 2,6-dimethyl-4-hydroxybenzoic acid) is presented. A common feature of these structures are parallel channels that are able to accommodate appropriately sized guest molecules. The structures differ with respect to the steric congestion within the channels arising from methyl groups appended to the bridging ligands of the network. The host network, Zn(2-Mehba), is able to take up appreciable quantities of H2 (77 K) and CO2 and CH4 (298 K) in a reversible manner. In regard to the adsorption of N2 by Zn(2-Mehba), there appears to be an unusual temperature dependence for the uptake of the gas such that when the temperature is increased from 77 to 298 K the uptake of N2 increases. The relatively narrow channels of Zn(2,6-Me2hba) are too small to allow the uptake of N2 and CH4, but H2 molecules can be adsorbed. A pronounced step at elevated pressures in CO2 and N2O isotherms for Zn(2,6-Me2hba) is noted. Calculations indicate that rotation of phenolate rings leads to a change in the available intraframework space during CO2 dosing.
中文翻译:

立体活性配体取代基对3D Zn基配位聚合物家族中气体吸附的影响。
两种结构相关的3D配位聚合物Zn(2-Mehba)和Zn(2,6-Me 2 hba)(2-Mehba = 2-甲基-4-羟基苯甲酸的二价阴离子和给出了2,6-Me 2 hba = 2,6-二甲基-4-羟基苯甲酸的二价阴离子。这些结构的共同特征是能够容纳适当大小的客体分子的平行通道。就通道内的空间拥挤而言,结构有所不同,该通道是由连接至网络桥接配体的甲基所引起的。主机网络Zn(2-Mehba)能够吸收相当数量的H 2(77 K)以及CO 2和CH 4(298 K)以可逆的方式。关于Zn(2-Mehba)对N 2的吸附,对于气体的吸收似乎存在不寻常的温度依赖性,使得当温度从77 K增加到298 K时,N 2的吸收增加。Zn(2,6-Me 2 hba)的相对较窄的通道太小,无法吸收N 2和CH 4,但是H 2分子可以被吸附。注意到在Zn(2,6-Me 2 hba)的CO 2和N 2 O等温线升高的压力下有一个明显的步骤。计算表明,酚盐环的旋转导致CO 2期间可用的框架内空间发生变化 加药。
更新日期:2020-07-06
中文翻译:

立体活性配体取代基对3D Zn基配位聚合物家族中气体吸附的影响。
两种结构相关的3D配位聚合物Zn(2-Mehba)和Zn(2,6-Me 2 hba)(2-Mehba = 2-甲基-4-羟基苯甲酸的二价阴离子和给出了2,6-Me 2 hba = 2,6-二甲基-4-羟基苯甲酸的二价阴离子。这些结构的共同特征是能够容纳适当大小的客体分子的平行通道。就通道内的空间拥挤而言,结构有所不同,该通道是由连接至网络桥接配体的甲基所引起的。主机网络Zn(2-Mehba)能够吸收相当数量的H 2(77 K)以及CO 2和CH 4(298 K)以可逆的方式。关于Zn(2-Mehba)对N 2的吸附,对于气体的吸收似乎存在不寻常的温度依赖性,使得当温度从77 K增加到298 K时,N 2的吸收增加。Zn(2,6-Me 2 hba)的相对较窄的通道太小,无法吸收N 2和CH 4,但是H 2分子可以被吸附。注意到在Zn(2,6-Me 2 hba)的CO 2和N 2 O等温线升高的压力下有一个明显的步骤。计算表明,酚盐环的旋转导致CO 2期间可用的框架内空间发生变化 加药。











































 京公网安备 11010802027423号
京公网安备 11010802027423号