当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acetylene/Ethylene Separation and Solid-State Structural Transformation via [2 + 2] Cycloaddition Reactions in 3D Microporous ZnII Metal-Organic Frameworks.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.inorgchem.0c00932 Arpan Hazra 1 , Aashima Jain 1 , M S Deenadayalan 1 , Stephen Adie Adalikwu 1 , Tapas Kumar Maji 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.inorgchem.0c00932 Arpan Hazra 1 , Aashima Jain 1 , M S Deenadayalan 1 , Stephen Adie Adalikwu 1 , Tapas Kumar Maji 1
Affiliation
|
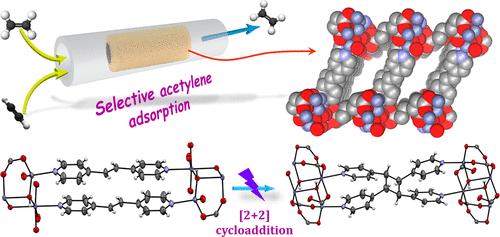
Purification of ethylene by removing acetylene from an ethylene/acetylene (99/1 v/v) mixture is a challenging task in the petrochemical industry. Our effort toward finding new porous materials that can selectively uptake acetylene resulted in two 3D metal–organic frameworks. Compound 1, {[Zn(p-pda)(bpee)]} (p-pda = p-phenylenediacetate and bpee = 1,2-bis(4-pyridyl)ethylene) turned out to be a nonporous structure due to the presence of a bulky and rigid aromatic ring in the p-pda ligand. Interestingly, replacement of p-pda with succinic acid yields the microporous framework {[Zn2(μ3-OH)(suc)1.5(bpee)]·CH3OH·2H2O} (2; suc = succinate), which offers a 1D channel along the a direction occupied by guest methanol and water molecules. Remarkably, the desolvated framework of 2 shows selective uptake of C2H2 over other gas molecules such as C2H4, C2H6, CO2 and CH4 at 293 K. An ideal absorbed solution theory (IAST) study predicts a high selectivity value for acetylene adsorption over the other gas molecules mentioned above. The efficiency of removal of acetylene from ethylene/acetylene mixtures containing 1% acetylene was established through a column breakthrough experiment performed at room temperature. The performance of our material in the purification of ethylene by removing acetylene is comparable with those reported in the literature. In the frameworks of both 1 and 2, the ethylenic double bonds of adjacent bpee linkers are aligned parallel and readily undergo [2 + 2] cycloaddition reactions upon UV-light irradiation to yield {[Zn2(p-pda)2(rctt-tpcb)]} (1IR) and {[Zn4(μ3-OH)2(suc)3(rctt-tpcb)]·2CH3OH·4H2O} (2IR), respectively (rctt-tpcb = regio-cis,trans,trans-tetrakis(4-pyridyl)cyclobutane). Photochemical structural transformations of 100% were observed in single-crystal to single-crystal fashions, which were also supported by 1H NMR spectroscopy. The structures of both 2 and 2IR underwent temperature-dependent reversible structural transformations, which was confirmed by SCXRD and DSC analysis. Selective C2H2 uptake of the dehydrated framework of 2IR was also examined, which demonstrates results similar to those of 2.
中文翻译:

在3D微孔ZnII金属有机骨架中通过[2 + 2]环加成反应进行乙炔/乙烯分离和固态结构转变。
通过从乙烯/乙炔(99/1 v / v)混合物中除去乙炔来纯化乙烯在石化行业中是一项艰巨的任务。我们努力寻找可以选择性吸收乙炔的新型多孔材料的努力产生了两个3D金属-有机骨架。由于存在,化合物1 {{Zn(p- pda)(bpee)]}(p- pda =对苯二乙酸酯和bpee = 1,2-双(4-吡啶基)乙烯)证明是无孔结构p- pda配体中大而刚性的芳香环的结构。有趣的是,替换的p -pda与琥珀酸的产率微孔骨架{[锌2(μ 3 -OH)(SUC)1.5(bpee)]·CH 3 OH·2H 2 O}(2; suc =琥珀酸酯),其沿客体甲醇和水分子占据的方向提供一维通道。显着地,2的去溶剂化骨架显示出C 2 H 2相对于其他气体分子(例如C 2 H 4,C 2 H 6,CO 2和CH 4)的选择性吸收在293 K时。理想吸收溶液理论(IAST)研究预测,乙炔对上述其他气体分子的吸附选择性高。通过在室温下进行的柱穿透实验,确定了从含有1%乙炔的乙烯/乙炔混合物中除去乙炔的效率。我们的材料通过去除乙炔提纯乙烯的性能可与文献报道相媲美。在1和2的框架中,相邻bpee连接子的烯键双键平行排列,在紫外线照射下易于进行[2 + 2]环加成反应,生成{[Zn 2(p -pda)2(rctt-tpcb)]}(1IR)和{[锌4(μ 3 -OH)2(SUC)3(rctt -tpcb)]·2CH 3 OH·4H 2 ö}(2IR),分别为(rctt -tpcb =区域选择性-顺式,反式,反式-四(4-吡啶基)环丁烷)。以单晶到单晶的方式观察到100%的光化学结构转变,这也得到1 H NMR光谱的支持。两者的结构2和2IR后行依赖于温度的可逆的结构变换,将其通过SCXRD和DSC分析确认。选择性C还检查了2IR脱水骨架对2 H 2的吸收,这表明结果与2相似。
更新日期:2020-07-06
中文翻译:

在3D微孔ZnII金属有机骨架中通过[2 + 2]环加成反应进行乙炔/乙烯分离和固态结构转变。
通过从乙烯/乙炔(99/1 v / v)混合物中除去乙炔来纯化乙烯在石化行业中是一项艰巨的任务。我们努力寻找可以选择性吸收乙炔的新型多孔材料的努力产生了两个3D金属-有机骨架。由于存在,化合物1 {{Zn(p- pda)(bpee)]}(p- pda =对苯二乙酸酯和bpee = 1,2-双(4-吡啶基)乙烯)证明是无孔结构p- pda配体中大而刚性的芳香环的结构。有趣的是,替换的p -pda与琥珀酸的产率微孔骨架{[锌2(μ 3 -OH)(SUC)1.5(bpee)]·CH 3 OH·2H 2 O}(2; suc =琥珀酸酯),其沿客体甲醇和水分子占据的方向提供一维通道。显着地,2的去溶剂化骨架显示出C 2 H 2相对于其他气体分子(例如C 2 H 4,C 2 H 6,CO 2和CH 4)的选择性吸收在293 K时。理想吸收溶液理论(IAST)研究预测,乙炔对上述其他气体分子的吸附选择性高。通过在室温下进行的柱穿透实验,确定了从含有1%乙炔的乙烯/乙炔混合物中除去乙炔的效率。我们的材料通过去除乙炔提纯乙烯的性能可与文献报道相媲美。在1和2的框架中,相邻bpee连接子的烯键双键平行排列,在紫外线照射下易于进行[2 + 2]环加成反应,生成{[Zn 2(p -pda)2(rctt-tpcb)]}(1IR)和{[锌4(μ 3 -OH)2(SUC)3(rctt -tpcb)]·2CH 3 OH·4H 2 ö}(2IR),分别为(rctt -tpcb =区域选择性-顺式,反式,反式-四(4-吡啶基)环丁烷)。以单晶到单晶的方式观察到100%的光化学结构转变,这也得到1 H NMR光谱的支持。两者的结构2和2IR后行依赖于温度的可逆的结构变换,将其通过SCXRD和DSC分析确认。选择性C还检查了2IR脱水骨架对2 H 2的吸收,这表明结果与2相似。











































 京公网安备 11010802027423号
京公网安备 11010802027423号