当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-Aminooxazole as a Novel Privileged Scaffold in Antitubercular Medicinal Chemistry.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-06-08 , DOI: 10.1021/acsmedchemlett.0c00173 Elisa Azzali 1, 1 , Miriam Girardini 1, 1 , Giannamaria Annunziato 1, 1 , Marialaura Pavone 1, 1 , Federica Vacondio 1, 2 , Giorgia Mori 3 , Maria Rosalia Pasca 3 , Gabriele Costantino 1, 1, 2, 4 , Marco Pieroni 1, 1, 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-06-08 , DOI: 10.1021/acsmedchemlett.0c00173 Elisa Azzali 1, 1 , Miriam Girardini 1, 1 , Giannamaria Annunziato 1, 1 , Marialaura Pavone 1, 1 , Federica Vacondio 1, 2 , Giorgia Mori 3 , Maria Rosalia Pasca 3 , Gabriele Costantino 1, 1, 2, 4 , Marco Pieroni 1, 1, 2
Affiliation
|
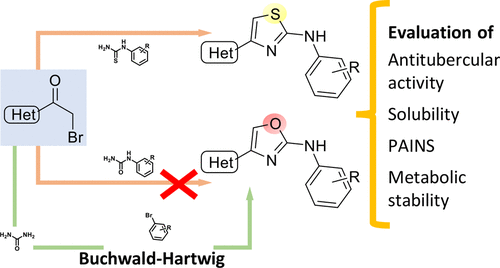
To obtain effective eradication of numerous infectious diseases such as tuberculosis, it is important to supply the medicinal chemistry arsenal with novel chemical agents. Isosterism and bioisosterism are widely known concepts in the field of early drug discovery, and in several cases, rational isosteric replacements have contributed to improved efficacy and physicochemical characteristics throughout the hit-to-lead optimization process. However, sometimes the synthesis of isosteres might not be as straightforward as that of the parent compounds, and therefore, novel synthetic strategies must be elaborated. In this regard, we herein report the evaluation of a series of N-substituted 4-phenyl-2-aminooxazoles that, despite being isosteres of a widely used nucleus such as the 2-aminothiazole, have been only seldom explored. After elaboration of a convenient synthetic strategy, a small set of 2-aminothiazoles and their 2-aminooxazole counterparts were compared with regard to antitubercular activity and physicochemical characteristics.
中文翻译:

2-氨基恶唑是抗结核药物化学中的新型特权支架。
为了有效地根除许多传染病,例如结核病,重要的是向药物化学武器库提供新型化学试剂。在早期药物发现领域中,等位基因和生物等位基因是广为人知的概念,在某些情况下,合理的等位基因替代已在从铅到铅的最优化过程中改善了功效和理化特性。但是,有时等位基因的合成可能不像母体化合物那样简单,因此,必须详细阐述新的合成策略。在这方面,我们在本文中报道了对一系列N-取代的4-苯基-2-氨基恶唑的评价,尽管它们是诸如2-氨基噻唑的广泛使用的核的等排体,但很少被探索。
更新日期:2020-07-09
中文翻译:

2-氨基恶唑是抗结核药物化学中的新型特权支架。
为了有效地根除许多传染病,例如结核病,重要的是向药物化学武器库提供新型化学试剂。在早期药物发现领域中,等位基因和生物等位基因是广为人知的概念,在某些情况下,合理的等位基因替代已在从铅到铅的最优化过程中改善了功效和理化特性。但是,有时等位基因的合成可能不像母体化合物那样简单,因此,必须详细阐述新的合成策略。在这方面,我们在本文中报道了对一系列N-取代的4-苯基-2-氨基恶唑的评价,尽管它们是诸如2-氨基噻唑的广泛使用的核的等排体,但很少被探索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号