当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Macrocyclic Iminopeptides Diversify To Better Target Proteins.
ChemMedChem ( IF 3.4 ) Pub Date : 2020-06-09 , DOI: 10.1002/cmdc.202000261 Leslie Reguera 1 , Daniel G Rivera 1
ChemMedChem ( IF 3.4 ) Pub Date : 2020-06-09 , DOI: 10.1002/cmdc.202000261 Leslie Reguera 1 , Daniel G Rivera 1
Affiliation
|
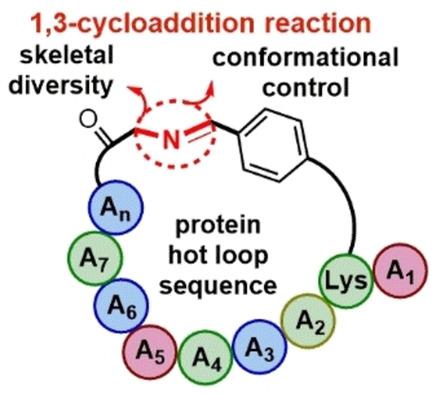
Among the many methods available for accessing conformationally diverse cyclic peptides, the derivatization of macrocyclic iminopeptides has remained notably underexplored. Now, a relevant complexity‐generating method expands the repertoire of synthetic strategies exploiting the reactivity of an imino bond embedded in the cyclic peptide skeleton. Here we highlight a recent report describing the on‐resin construction of a new family of macrocyclic peptide/natural product‐inspired hybrids, namely “PepNats”, by derivatization of cyclic iminopeptides through 1,3‐cycloaddition reactions. A proof‐of‐concept with PepNats bearing peptide sequences that mimic protein hot loops demonstrated the potential of this strategy to create novel macrocyclic peptide ligands capable of modulating protein–protein interactions.
中文翻译:

大环亚肽多样化以更好地靶向蛋白质。
在可用于访问构象多样的环肽的许多方法中,大环亚氨基肽的衍生化仍显着不足。现在,一种相关的复杂度生成方法利用嵌入在环肽骨架中的亚氨基键的反应性,扩大了合成策略的范围。在这里,我们重点介绍了最近的一份报告,该报告描述了通过1,3-环加成反应使环亚氨基肽衍生化的大环肽/天然产物启发的杂种的新家族,即“ PepNats”的树脂构建。PepNats带有模拟蛋白热环的肽序列的概念验证证明了该策略潜在的潜力,可以创建能够调节蛋白与蛋白相互作用的新型大环肽配体。
更新日期:2020-07-03
中文翻译:

大环亚肽多样化以更好地靶向蛋白质。
在可用于访问构象多样的环肽的许多方法中,大环亚氨基肽的衍生化仍显着不足。现在,一种相关的复杂度生成方法利用嵌入在环肽骨架中的亚氨基键的反应性,扩大了合成策略的范围。在这里,我们重点介绍了最近的一份报告,该报告描述了通过1,3-环加成反应使环亚氨基肽衍生化的大环肽/天然产物启发的杂种的新家族,即“ PepNats”的树脂构建。PepNats带有模拟蛋白热环的肽序列的概念验证证明了该策略潜在的潜力,可以创建能够调节蛋白与蛋白相互作用的新型大环肽配体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号