当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Variations in Proteins Dielectric Constants.
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-06-08 , DOI: 10.1002/open.202000108 Muhamed Amin 1, 2, 3 , Jochen Küpper 1, 4, 5
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-06-08 , DOI: 10.1002/open.202000108 Muhamed Amin 1, 2, 3 , Jochen Küpper 1, 4, 5
Affiliation
|
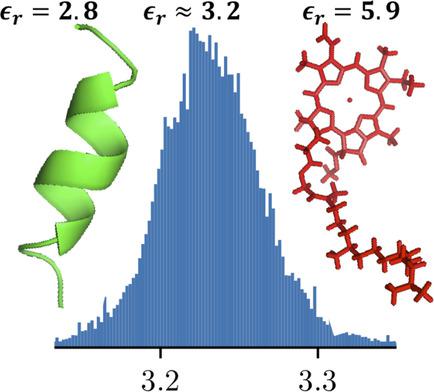
Using a new semi‐empirical method for calculating molecular polarizabilities and the Clausius−Mossotti relation, we calculated the static dielectric constants of dry proteins for all structures in the protein data bank (PDB). The mean dielectric constant of more than 150,000 proteins is  with a standard deviation of 0.04, which agrees well with previous measurement for dry proteins. The small standard deviation results from the strong correlation between the molecular polarizability and the volume of the proteins. We note that non‐amino acid cofactors such as Chlorophyll may alter the dielectric environment significantly. Furthermore, our model shows anisotropies of the dielectric constant within the same molecule according to the constituents amino acids and cofactors. Finally, by changing the amino acid protonation states, we show that a change of pH does not have a significant effect on the dielectric constants of proteins.
with a standard deviation of 0.04, which agrees well with previous measurement for dry proteins. The small standard deviation results from the strong correlation between the molecular polarizability and the volume of the proteins. We note that non‐amino acid cofactors such as Chlorophyll may alter the dielectric environment significantly. Furthermore, our model shows anisotropies of the dielectric constant within the same molecule according to the constituents amino acids and cofactors. Finally, by changing the amino acid protonation states, we show that a change of pH does not have a significant effect on the dielectric constants of proteins.
中文翻译:

蛋白质介电常数的变化。
使用一种新的半经验方法来计算分子极化率和 Clausius−Mossotti 关系,我们计算了蛋白质数据库 (PDB) 中所有结构的干蛋白质的静态介电常数。超过 150,000 种蛋白质的平均介电常数标准差 为 0.04,这与之前对干蛋白质的测量结果非常吻合。小标准偏差是由于分子极化率和蛋白质体积之间的强相关性造成的。我们注意到,叶绿素等非氨基酸辅助因子可能会显着改变介电环境。此外,我们的模型根据氨基酸和辅因子的组成显示了同一分子内介电常数的各向异性。最后,通过改变氨基酸质子化状态,我们表明 pH 值的变化对蛋白质的介电常数没有显着影响。
为 0.04,这与之前对干蛋白质的测量结果非常吻合。小标准偏差是由于分子极化率和蛋白质体积之间的强相关性造成的。我们注意到,叶绿素等非氨基酸辅助因子可能会显着改变介电环境。此外,我们的模型根据氨基酸和辅因子的组成显示了同一分子内介电常数的各向异性。最后,通过改变氨基酸质子化状态,我们表明 pH 值的变化对蛋白质的介电常数没有显着影响。
更新日期:2020-06-08
 with a standard deviation of 0.04, which agrees well with previous measurement for dry proteins. The small standard deviation results from the strong correlation between the molecular polarizability and the volume of the proteins. We note that non‐amino acid cofactors such as Chlorophyll may alter the dielectric environment significantly. Furthermore, our model shows anisotropies of the dielectric constant within the same molecule according to the constituents amino acids and cofactors. Finally, by changing the amino acid protonation states, we show that a change of pH does not have a significant effect on the dielectric constants of proteins.
with a standard deviation of 0.04, which agrees well with previous measurement for dry proteins. The small standard deviation results from the strong correlation between the molecular polarizability and the volume of the proteins. We note that non‐amino acid cofactors such as Chlorophyll may alter the dielectric environment significantly. Furthermore, our model shows anisotropies of the dielectric constant within the same molecule according to the constituents amino acids and cofactors. Finally, by changing the amino acid protonation states, we show that a change of pH does not have a significant effect on the dielectric constants of proteins.
中文翻译:

蛋白质介电常数的变化。
使用一种新的半经验方法来计算分子极化率和 Clausius−Mossotti 关系,我们计算了蛋白质数据库 (PDB) 中所有结构的干蛋白质的静态介电常数。超过 150,000 种蛋白质的平均介电常数标准差
 为 0.04,这与之前对干蛋白质的测量结果非常吻合。小标准偏差是由于分子极化率和蛋白质体积之间的强相关性造成的。我们注意到,叶绿素等非氨基酸辅助因子可能会显着改变介电环境。此外,我们的模型根据氨基酸和辅因子的组成显示了同一分子内介电常数的各向异性。最后,通过改变氨基酸质子化状态,我们表明 pH 值的变化对蛋白质的介电常数没有显着影响。
为 0.04,这与之前对干蛋白质的测量结果非常吻合。小标准偏差是由于分子极化率和蛋白质体积之间的强相关性造成的。我们注意到,叶绿素等非氨基酸辅助因子可能会显着改变介电环境。此外,我们的模型根据氨基酸和辅因子的组成显示了同一分子内介电常数的各向异性。最后,通过改变氨基酸质子化状态,我们表明 pH 值的变化对蛋白质的介电常数没有显着影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号