当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Repurposing a bacterial prolidase for organophosphorus hydrolysis: Reshaped catalytic cavity switches substrate selectivity.
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-09 , DOI: 10.1002/bit.27455 Jian Yang 1, 2 , Yun-Zhu Xiao 1, 3 , Ru Li 1, 4 , Yu Liu 1, 4 , Li-Juan Long 1, 2, 4
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-09 , DOI: 10.1002/bit.27455 Jian Yang 1, 2 , Yun-Zhu Xiao 1, 3 , Ru Li 1, 4 , Yu Liu 1, 4 , Li-Juan Long 1, 2, 4
Affiliation
|
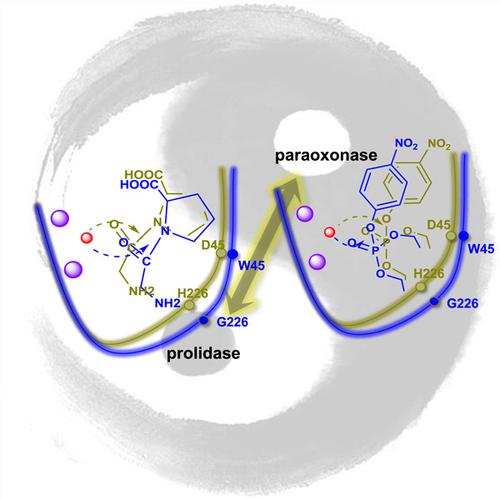
Enzyme promiscuity is critical to the acquisition of evolutionary plasticity in cells and can be recruited for high‐value chemical synthesis or xenobiotic degradation. The molecular determinants of substrate ambiguity are essential to this activity; however, these details remain unknown. Here, we performed the directed evolution of a prolidase to enhance its initially weak paraoxonase activity. The in vitro evolution led to an unexpected 1,000,000‐fold switch in substrate selectivity, with a 30‐fold increase in paraoxon hydrolysis and 40,000‐fold decrease in peptide hydrolysis. Structural and in silico analyses revealed enlarged catalytic cavities and substrate repositioning as responsible for rapid catalytic transitions between distinct chemical reactions.
中文翻译:

重新利用细菌脯氨酸酶进行有机磷水解:重塑催化腔转换底物选择性。
酶混杂对于获得细胞进化可塑性至关重要,可用于高价值化学合成或异生物质降解。底物歧义的分子决定因素对这项活动至关重要;然而,这些细节仍然未知。在这里,我们对脯氨酸酶进行了定向进化,以增强其最初较弱的对氧磷酶活性。体外进化导致底物选择性发生意外的 1,000,000 倍转换,对氧磷水解增加 30 倍,肽水解减少 40,000 倍。结构和计算机分析显示,扩大的催化腔和底物重新定位是导致不同化学反应之间快速催化转变的原因。
更新日期:2020-08-14
中文翻译:

重新利用细菌脯氨酸酶进行有机磷水解:重塑催化腔转换底物选择性。
酶混杂对于获得细胞进化可塑性至关重要,可用于高价值化学合成或异生物质降解。底物歧义的分子决定因素对这项活动至关重要;然而,这些细节仍然未知。在这里,我们对脯氨酸酶进行了定向进化,以增强其最初较弱的对氧磷酶活性。体外进化导致底物选择性发生意外的 1,000,000 倍转换,对氧磷水解增加 30 倍,肽水解减少 40,000 倍。结构和计算机分析显示,扩大的催化腔和底物重新定位是导致不同化学反应之间快速催化转变的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号