Tetrahedron ( IF 2.1 ) Pub Date : 2020-06-09 , DOI: 10.1016/j.tet.2020.131337 Urvashi , Mohammad Ovais Dar , Prasad V. Bharatam , Parthasarathi Das , Shrikant Kukreti , Vibha Tandon
|
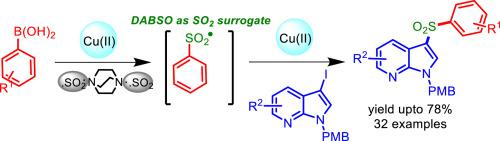
DABSO mediated sulfonylation of iodinated 7-azaindoles was achieved for the first time through sulfonylative Suzuki-Miyaura cross coupling (SMC) reaction under mild conditions giving good yields of sulfonylated 7-azaindole derivatives. Interestingly, control experiments suggest that present method involves in-situ generation of ArSO2 free radical followed by the key steps of SMC reaction. Scope of the reaction was explored with both electronically different and bulky group carrying boronic acids as coupling partner. The sulfonylation is scalable and occurred selectively at iodo group, irrespective of its position on azaindole. Moreover, the proposed mechanism has been supported by electron paramagnetic resonance (EPR) and density functional theory (DFT) calculations.
中文翻译:

铜(II)-DABSO作为SO 2催化7-氮杂吲哚磺酰化反应及其机理研究
在温和的条件下,通过磺化的Suzuki-Miyaura交叉偶联(SMC)反应首次实现了DABSO介导的碘化7-氮杂吲哚的磺酰化反应,得到了磺酰化的7-氮杂吲哚衍生物的良好收率。有趣的是,对照实验表明,本方法涉及原位生成ArSO 2自由基,然后进行SMC反应的关键步骤。以电子上不同的和带有硼酸作为偶联伙伴的大体积基团探索了反应的范围。磺酰化是可扩展的并且选择性地发生在碘基团上,而不管其在氮杂吲哚上的位置。此外,提出的机制已得到电子顺磁共振(EPR)和密度泛函理论(DFT)计算的支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号