Structure ( IF 4.4 ) Pub Date : 2020-06-09 , DOI: 10.1016/j.str.2020.05.010 Samual D Dick 1 , Stefania Federico 2 , Siobhan M Hughes 1 , Valerie E Pye 1 , Nicola O'Reilly 2 , Peter Cherepanov 3
|
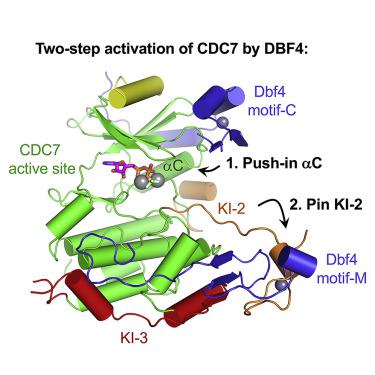
CDC7 is an essential Ser/Thr kinase that acts upon the replicative helicase throughout the S phase of the cell cycle and is activated by DBF4. Here, we present crystal structures of a highly active human CDC7-DBF4 construct. The structures reveal a zinc-finger domain at the end of the kinase insert 2 that pins the CDC7 activation loop to motif M of DBF4 and the C lobe of CDC7. These interactions lead to ordering of the substrate-binding platform and full opening of the kinase active site. In a co-crystal structure with a mimic of MCM2 Ser40 phosphorylation target, the invariant CDC7 residues Arg373 and Arg380 engage phospho-Ser41 at substrate P+1 position, explaining the selectivity of the S-phase kinase for Ser/Thr residues followed by a pre-phosphorylated or an acidic residue. Our results clarify the role of DBF4 in activation of CDC7 and elucidate the structural basis for recognition of its preferred substrates.
中文翻译:

CDC7激酶激活和靶位点特异性的结构基础。
CDC7 是一种必需的 Ser/Thr 激酶,在整个细胞周期的 S 期作用于复制解旋酶,并被 DBF4 激活。在这里,我们展示了一种高活性人类 CDC7-DBF4 构建体的晶体结构。这些结构揭示了激酶插入物 2 末端的锌指结构域,该结构域将 CDC7 激活环固定在 DBF4 的基序 M 和 CDC7 的 C 叶上。这些相互作用导致底物结合平台的排序和激酶活性位点的完全开放。在具有 MCM2 Ser40 磷酸化靶标模拟物的共晶体结构中,不变的 CDC7 残基 Arg373 和 Arg380 在底物 P+1 位置与磷酸化 Ser41 结合,这解释了 S 期激酶对 Ser/Thr 残基的选择性,然后是预磷酸化或酸性残基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号