当前位置:
X-MOL 学术
›
Comp. Mater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular dynamics simulations of zinc sulfide deposition on silver sulfide from aqueous solution
Computational Materials Science ( IF 3.1 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.commatsci.2020.109821 S.I. Sadovnikov , I.A. Balyakin
Computational Materials Science ( IF 3.1 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.commatsci.2020.109821 S.I. Sadovnikov , I.A. Balyakin
|
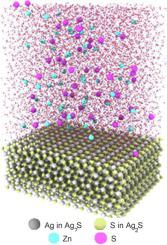
Abstract Classical molecular dynamics (CMD) simulation has been performed to study the ZnS deposition from aqueous solution on a surface of crystalline Ag2S. The initial configuration of the Ag2S + ZnS + H2O simulated system includes the surface of crystalline Ag2S silver sulfide with H2O molecules above this surface and Zn2+ and S2- ions located between water molecules. In Ag2S + ZnS + H2O system, sulfur ions are adsorbed originally from a solution on Ag2S surface. The part of deposited Zn atoms Zn is under the first deposited layer of S atoms S, and other Zn atoms Zn are located approximately level with surface S atoms. Performed CMD calculation has shown that the dependences of relative number of the deposited sulfur and zinc atoms on the deposition time are symbate, and formation of first surface ZnS layer occurs during deposition about 10 ns. In addition, the analytical model of ZnS deposition on Ag2S surface is proposed. The CMD simulation confirmed the adequacy of this analytical model.
中文翻译:

硫化锌在水溶液中硫化银沉积的分子动力学模拟
摘要 已进行经典分子动力学 (CMD) 模拟以研究水溶液中 ZnS 在结晶 Ag2S 表面的沉积。Ag2S + ZnS + H2O 模拟系统的初始配置包括结晶 Ag2S 硫化银表面,H2O 分子在该表面上方,Zn2+ 和 S2- 离子位于水分子之间。在 Ag2S + ZnS + H2O 系统中,硫离子最初是从 Ag2S 表面的溶液中吸附的。沉积的部分Zn原子Zn位于S原子S的第一沉积层之下,其他Zn原子Zn位于与表面S原子大致齐平的位置。进行的 CMD 计算表明,沉积的硫和锌原子的相对数量与沉积时间的相关性是一致的,第一表面 ZnS 层的形成发生在大约 10 ns 的沉积过程中。此外,还提出了ZnS在Ag2S表面沉积的解析模型。CMD 模拟证实了该分析模型的充分性。
更新日期:2020-11-01
中文翻译:

硫化锌在水溶液中硫化银沉积的分子动力学模拟
摘要 已进行经典分子动力学 (CMD) 模拟以研究水溶液中 ZnS 在结晶 Ag2S 表面的沉积。Ag2S + ZnS + H2O 模拟系统的初始配置包括结晶 Ag2S 硫化银表面,H2O 分子在该表面上方,Zn2+ 和 S2- 离子位于水分子之间。在 Ag2S + ZnS + H2O 系统中,硫离子最初是从 Ag2S 表面的溶液中吸附的。沉积的部分Zn原子Zn位于S原子S的第一沉积层之下,其他Zn原子Zn位于与表面S原子大致齐平的位置。进行的 CMD 计算表明,沉积的硫和锌原子的相对数量与沉积时间的相关性是一致的,第一表面 ZnS 层的形成发生在大约 10 ns 的沉积过程中。此外,还提出了ZnS在Ag2S表面沉积的解析模型。CMD 模拟证实了该分析模型的充分性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号