Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2020-06-09 , DOI: 10.1016/j.bbapap.2020.140468 Deepika Prasad 1 , Kalappa Muniyappa 1
|
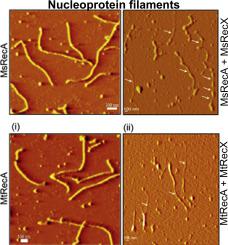
The members of the RecX family of proteins have a unique capacity to regulate the catalytic activities of RecA/Rad51 proteins in both prokaryotic and eukaryotic organisms. However, our understanding of the functional roles of RecX in pathogenic and non-pathogenic mycobacteria has been limited by insufficient knowledge of the molecular mechanisms of its activity and regulation. Moreover, the significance of a unique 14 amino acid N-terminal extension in Mycobacterium smegmatis RecX (MsRecX) to its function remains unknown. Here, we advance our understanding of the antagonistic roles of mycobacterial RecX proteins and the functional significance of the extended N-terminus of MsRecX. The full-length MsRecX acts as an antagonist of RecA, negatively regulating RecA promoted functions, including DNA strand exchange, LexA cleavage and ATP hydrolysis, but not binding of ATP. The N-terminally truncated MsRecX variants retain the RecA inhibitory activity, albeit with lower efficiencies compared to the full-length protein. Perhaps most importantly, direct visualization of RecA nucleoprotein filaments, which had been incubated with RecX proteins, showed that they promote disassembly of nucleoprotein filaments primarily within the filaments. In addition, interaction of RecX proteins with the RecA nucleoprotein filaments results in the formation of stiff and irregularly shaped nucleoprotein filaments. Thus, these findings add an additional mechanism by which RecX disassembles RecA nucleoprotein filaments. Overall, this study provides strong evidence for the notion that the N-terminal 14 amino acid region of MsRecX plays an important role in the negative regulation of RecA functions and new insights into the molecular mechanism underlying RecX function.
中文翻译:

耻垢分枝杆菌RecX的扩展N末端增强了其对抗RecA功能的能力。
RecX蛋白家族的成员具有独特的能力来调节原核和真核生物中RecA / Rad51蛋白的催化活性。但是,我们对RecX在致病性和非致病性分枝杆菌中的功能作用的理解受到了对其活性和调控的分子机制了解不足而受到限制。此外,耻垢分枝杆菌中独特的14个氨基酸的N末端延伸的意义RecX(MsRecX)的功能仍然未知。在这里,我们进一步了解分枝杆菌RecX蛋白的拮抗作用以及MsRecX扩展N端的功能意义。全长MsRecX充当RecA的拮抗剂,负调节RecA促进的功能,包括DNA链交换,LexA裂解和ATP水解,但不与ATP结合。Ns端截短的MsRecX变体保留了RecA抑制活性,尽管与全长蛋白相比效率较低。也许最重要的是,与RecX蛋白质一起孵育的RecA核蛋白细丝的直接可视化显示,它们主要促进细丝内的核蛋白细丝的分解。此外,RecX蛋白与RecA核蛋白丝之间的相互作用会导致形成刚性且形状不规则的核蛋白丝。因此,这些发现增加了RecX拆卸RecA核蛋白丝的另一种机制。总体而言,这项研究为以下观点提供了有力证据:MsRecX的N末端14个氨基酸区域在RecA功能的负调控中起着重要作用,并为RecC功能的分子机制提供了新见解。










































 京公网安备 11010802027423号
京公网安备 11010802027423号