Current Computer-Aided Drug Design ( IF 1.5 ) Pub Date : 2021-06-30 , DOI: 10.2174/1573409916666200525150410 Divya Chauhan 1 , Sushil Kumar 2 , Syed Riaz Hashim 1 , Vinit Raj 3
|
Objective: The main objective of the study was to develop the Quantitative Structure- Activity Relationship (QSAR) and pharmacophore model by using data obtained from HT-29 cells to develop potent lead molecule for the scientific community.
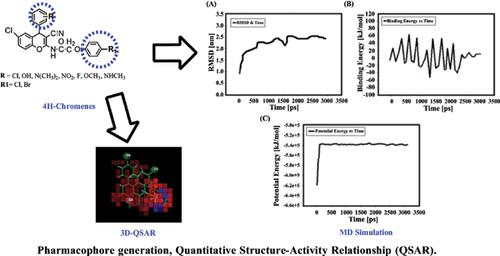
Materials and Methods: Common pharmacophore model, atom-based 3D-QSAR, and molecular dynamic (MD) simulation were carried out via computational techniques by using 4H-chromene derivatives.
Results: The reliable common pharmacophoric hypothesis, DHH13 was generated and 3.95 survival value was also found. Furthermore, the statistically significant 3D-QSAR model was developed where r2=0.52 was found by using the Partial least squares (PLS) regression method. Phase predicted activity and Log GI50 demonstrated an important atomic position in the structure of ligands to ascertain anti colon cancer activity. Also, MD simulation was carried out between top rank leads targeting IL-6 that provided better binding conformational and complex stability into the active pocket site of the target throughout the MD simulation.
Conclusion: The outcome of this design shows that the pharmacophore model and 3D-QSAR might be helpful for researchers in the field of medicinal chemistry to design and develop potential anti colon cancer compounds.
中文翻译:

新取代的 N-(6- Chloro-3-cyano-4-phenyl-4H-chromen-2-yl)-2-(4-chloro-) 的药效团生成、定量构效关系 (QSAR) 和分子动力学模拟苯氧基)-乙酰胺抗癌活性
目的:该研究的主要目的是利用从 HT-29 细胞获得的数据开发定量构效关系 (QSAR) 和药效团模型,从而为科学界开发有效的先导分子。
材料和方法:使用 4H-色烯衍生物通过计算技术进行常见药效团模型、基于原子的 3D-QSAR 和分子动力学 (MD) 模拟。
结果:产生了可靠的共同药效学假设DHH13,并发现了3.95的生存值。此外,通过使用偏最小二乘(PLS)回归方法发现了具有统计学意义的3D-QSAR模型,其中r 2 =0.52。相位预测活性和 Log GI 50证明了配体结构中的重要原子位置,以确定抗结肠癌活性。此外,在针对 IL-6 的顶级先导之间进行了 MD 模拟,在整个 MD 模拟过程中,IL-6 提供了更好的结合构象和复杂稳定性到目标的活性口袋位点。
结论:该设计的结果表明,药效团模型和 3D-QSAR 可能有助于药物化学领域的研究人员设计和开发潜在的抗结肠癌化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号