当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interplay among the "flipping" glutamine, a conserved phenylalanine, water and hydrogen bonds within a blue-light sensing LOV domain.
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-06-08 , DOI: 10.1039/d0pp00082e Eugenia Polverini 1 , Florian Karl Schackert 1, 2 , Aba Losi 1
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-06-08 , DOI: 10.1039/d0pp00082e Eugenia Polverini 1 , Florian Karl Schackert 1, 2 , Aba Losi 1
Affiliation
|
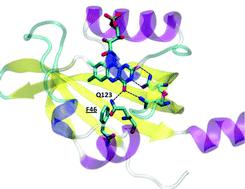
In this work we exploited time-resolved photoacoustics (PA) and molecular dynamics (MD) simulations to investigate the function of a conserved phenylalanine residue in blue sensing (BL) LOV domains. The LOV photocycle involves reversible formation of a photoproduct (LOV390) where the flavin mononucleotide (FMN) chromophore is covalently bound to a cysteine. LOV390 thermally returns to the dark adapted state (LOV447) with a lifetime τrec (s-to-h). In the LOV domain of Bacillus subtilis BsYtvA, the conserved F46 is one of the few residues undergoing a pronounced light-driven conformational change. PA and spectroscopic data show that in the YtvA variants F46A and F46Y light-induced structural changes are much smaller than those in the wild type (wt) protein, τrec is strongly accelerated and the energy content of LOV390 is lower for F46Y. MD simulations for each variant in the LOV447 and LOV390 states revealed an overall very stable structure of the BsYtvA-LOV domain. The largest variations emerged for the conserved HB network that includes FMN, Q123 (the “flipping” glutamine of LOV domains), and the conserved N104 and N94, with strong dependence on the presence of water. The lateral chain of Q123 in wt-LOV447 can adopt three alternative conformations, and movements act in concert with F46 flexibility. In LOV390, Q123 remains instead fixed in the orientation adopted in the crystal structure. Interestingly, in F46A, Q123 is locked in a LOV447-like conformation (pseudo-dark-adapted state), in both LOV447 and LOV390. In LOV447 of F46Y the tyrosine hydroxyl group fixes a water molecule, which induces a Q123 conformation similar to wt-LOV390, i.e. a pseudo-photoproduct state. These pseudo-dark-adapted and photoproduct-like conformations of the Q123 sidechain may account for the strong acceleration of the photocycle in the two variants. Given the importance of the “flipping” glutamine in light-to-signal propagation in LOV proteins, the results presented here underscore a crucial structural and functional role of the conserved F46. MD results also indicate that F46 is not directly engaged in permeability of the FMN pocket, but is involved in solvent ordering and the formation of water bridges.
中文翻译:

蓝光感应LOV域中的“翻转”谷氨酰胺,保守的苯丙氨酸,水和氢键之间的相互作用。
在这项工作中,我们利用时间分辨的光声(PA)和分子动力学(MD)模拟来研究保守的苯丙氨酸残基在蓝色传感(BL)LOV域中的功能。LOV光循环涉及光产物(LOV 390)的可逆形成,其中黄素单核苷酸(FMN)发色团共价结合至半胱氨酸。LOV 390热返回到暗适应状态下(LOV 447用寿命)τ REC(S到H)。在枯草芽孢杆菌Bs的LOV域中YtvA,保守的F46是经历明显的光驱动构象变化的少数残基之一。PA和光谱数据表明,在变体YtvA和F46A F46Y光诱导的结构变化比在野生型(wt)蛋白,小得多τ REC强烈加速和LOV的能量含量390为F46Y更低。分子动力学模拟在LOV每个变种447和LOV 390国道出了一个整体非常稳定的结构,烧烤YtvA-LOV域。保守的HB网络出现了最大的变化,其中包括FMN,Q123(LOV域的“翻转”谷氨酰胺)以及保守的N104和N94,它们对水的依赖性非常强。wt-LOV 447中Q123的侧链可以采用三种替代构型,并且运动与F46的灵活性协调一致。在LOV 390中,Q123仍然固定为晶体结构中采用的方向。有趣的是,在F46A中,Q123在LOV 447和LOV 390中都被锁定为类似LOV 447的构型(伪暗适应状态)。在LOV 447中在F46Y中,酪氨酸羟基固定了水分子,该水分子诱导类似于wt-LOV 390的Q123构象,即伪光产物状态。Q123侧链的这些伪暗适应和类似光产物的构象可能解释了两个变体中光循环的强烈加速。鉴于“翻转”谷氨酰胺在LOV蛋白的光信号传播中的重要性,此处给出的结果强调了保守F46的关键结构和功能作用。MD结果还表明,F46不直接参与FMN袋的渗透性,而是参与溶剂有序化和水桥的形成。
更新日期:2020-07-15
中文翻译:

蓝光感应LOV域中的“翻转”谷氨酰胺,保守的苯丙氨酸,水和氢键之间的相互作用。
在这项工作中,我们利用时间分辨的光声(PA)和分子动力学(MD)模拟来研究保守的苯丙氨酸残基在蓝色传感(BL)LOV域中的功能。LOV光循环涉及光产物(LOV 390)的可逆形成,其中黄素单核苷酸(FMN)发色团共价结合至半胱氨酸。LOV 390热返回到暗适应状态下(LOV 447用寿命)τ REC(S到H)。在枯草芽孢杆菌Bs的LOV域中YtvA,保守的F46是经历明显的光驱动构象变化的少数残基之一。PA和光谱数据表明,在变体YtvA和F46A F46Y光诱导的结构变化比在野生型(wt)蛋白,小得多τ REC强烈加速和LOV的能量含量390为F46Y更低。分子动力学模拟在LOV每个变种447和LOV 390国道出了一个整体非常稳定的结构,烧烤YtvA-LOV域。保守的HB网络出现了最大的变化,其中包括FMN,Q123(LOV域的“翻转”谷氨酰胺)以及保守的N104和N94,它们对水的依赖性非常强。wt-LOV 447中Q123的侧链可以采用三种替代构型,并且运动与F46的灵活性协调一致。在LOV 390中,Q123仍然固定为晶体结构中采用的方向。有趣的是,在F46A中,Q123在LOV 447和LOV 390中都被锁定为类似LOV 447的构型(伪暗适应状态)。在LOV 447中在F46Y中,酪氨酸羟基固定了水分子,该水分子诱导类似于wt-LOV 390的Q123构象,即伪光产物状态。Q123侧链的这些伪暗适应和类似光产物的构象可能解释了两个变体中光循环的强烈加速。鉴于“翻转”谷氨酰胺在LOV蛋白的光信号传播中的重要性,此处给出的结果强调了保守F46的关键结构和功能作用。MD结果还表明,F46不直接参与FMN袋的渗透性,而是参与溶剂有序化和水桥的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号