当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards a generic understanding of oxygen evolution reaction kinetics in polymer electrolyte water electrolysis
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-06-08 , DOI: 10.1039/d0ee00673d Tobias Schuler 1, 2, 3, 4 , Taro Kimura 1, 2, 3, 4, 5 , Thomas J. Schmidt 1, 2, 3, 4, 6 , Felix N. Büchi 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-06-08 , DOI: 10.1039/d0ee00673d Tobias Schuler 1, 2, 3, 4 , Taro Kimura 1, 2, 3, 4, 5 , Thomas J. Schmidt 1, 2, 3, 4, 6 , Felix N. Büchi 1, 2, 3, 4
Affiliation
|
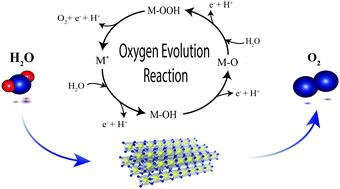
Water electrolysis is a key technology for future energy systems intended for the storage of fluctuating renewables and green industrial product upgrading. Under acidic electrolysis conditions, the oxygen evolution reaction (OER) predominantly causes the overpotential loss, which makes the elucidation of OER kinetics a task of key importance for future catalyst development. Herein, we design a methodology based on vapor-fed polymer electrolyte water electrolysis to fully characterize OER kinetics in the real environment of solid electrolytes and realize the controlled benchmarking of catalysts in the absence of gas passivation commonly observed in liquid-electrolyte half-cell configurations. Vapor-fed cells, allowing for distinct manipulation of water activity, sustain the essential degree of freedom to resolve and determine all four essential kinetic parameters. Thus, this work provides valuable insights into the OER mechanism, isolates the rate-determining step, and experimentally determines the reaction order of the state-of-the-art OER catalyst IrO2/TiO2 with respect to water. Through the combination of gas and liquid fed kinetic analysis, we elucidate the important missing link for formulating the generic governing relation for the OER overpotential and thereby providing a method for benchmarking of catalyst activities under technically representative conditions.
中文翻译:

对聚合物电解质水电解中析氧反应动力学的一般认识
水电解是未来能源系统的一项关键技术,旨在存储波动的可再生能源和绿色工业产品升级。在酸性电解条件下,析氧反应(OER)主要引起过电势损失,这使得阐明OER动力学成为未来催化剂开发的关键任务。在本文中,我们设计了一种基于气相填充聚合物电解质水电解的方法,以充分表征固体电解质实际环境中的OER动力学,并在不存在液体电解质半电池配置中常见的气体钝化的情况下实现催化剂的基准控制。蒸气补给的细胞,可以对水分活动进行独特的控制,维持解决和确定所有四个基本动力学参数的基本自由度。因此,这项工作为OER机理提供了有价值的见解,分离了速率确定步骤,并通过实验确定了最新OER催化剂IrO的反应顺序2 / TiO 2相对于水。通过气体和液体进料动力学分析的结合,我们阐明了为OER超电势建立通用控制关系的重要缺失环节,从而提供了在技术上具有代表性的条件下对催化剂活性进行标定的方法。
更新日期:2020-07-15
中文翻译:

对聚合物电解质水电解中析氧反应动力学的一般认识
水电解是未来能源系统的一项关键技术,旨在存储波动的可再生能源和绿色工业产品升级。在酸性电解条件下,析氧反应(OER)主要引起过电势损失,这使得阐明OER动力学成为未来催化剂开发的关键任务。在本文中,我们设计了一种基于气相填充聚合物电解质水电解的方法,以充分表征固体电解质实际环境中的OER动力学,并在不存在液体电解质半电池配置中常见的气体钝化的情况下实现催化剂的基准控制。蒸气补给的细胞,可以对水分活动进行独特的控制,维持解决和确定所有四个基本动力学参数的基本自由度。因此,这项工作为OER机理提供了有价值的见解,分离了速率确定步骤,并通过实验确定了最新OER催化剂IrO的反应顺序2 / TiO 2相对于水。通过气体和液体进料动力学分析的结合,我们阐明了为OER超电势建立通用控制关系的重要缺失环节,从而提供了在技术上具有代表性的条件下对催化剂活性进行标定的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号