当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonbonded Atomic Contacts Drive Ultrafast Helix Motions in Myoglobin.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jpcb.0c04772 Shinya Tahara 1 , Misao Mizuno 1 , Yasuhisa Mizutani 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jpcb.0c04772 Shinya Tahara 1 , Misao Mizuno 1 , Yasuhisa Mizutani 1
Affiliation
|
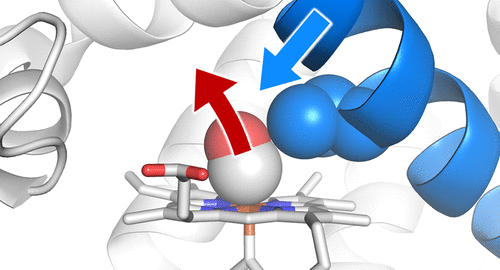
The association and dissociation of small ligands regulate the functions of proteins through structural changes in the protein. Such structural changes propagate long distances, and this allostery plays a key role in molecular functions. However, the mechanism by which structural changes are transmitted is poorly understood. Here we show that nonbonded atomic contacts play an essential role in driving the displacement of a helix in picosecond time scale primary structural changes following the dissociation of carbon monoxide from the heme group in myoglobin. The present time-resolved ultraviolet resonance Raman study revealed that the amplitude of this helix displacement was reduced upon substitution of Val68, which contacts the heme in wild-type myoglobin, with a less bulky side chain (Ala). Our findings provided the first direct evidence that structural changes are transmitted not only by covalent bonds, salt bridges and hydrogen bonds but also by nonbonded atomic contacts in the primary protein response upon ligand dissociation. Furthermore, the present results indicate the importance of dense atomic packing in a protein structure for responding to the association and dissociation of small molecules. The high compactness of protein structures makes possible the propagation of structural changes, providing useful clues to the design of molecular machines.
中文翻译:

非键合原子触点可驱动肌红蛋白中的超快螺旋运动。
小配体的缔合和解离通过蛋白质的结构变化调节蛋白质的功能。这种结构变化会传播很长的距离,并且这种构象在分子功能中起关键作用。但是,人们对传递结构变化的机制了解甚少。在这里,我们显示非结合的原子接触在皮秒级时域中的一氧化碳从肌红蛋白中的血红素基团解离后,在驱动螺旋位移的皮秒级尺度一级结构变化中起着至关重要的作用。目前的时间分辨紫外共振拉曼研究表明,该螺旋位移的幅度在取代Val68时降低,该Val68与野生型肌红蛋白中的血红素接触,但侧链较少(Ala)。我们的发现提供了第一个直接证据,表明结构变化不仅通过共价键,盐桥和氢键传递,而且还通过配体解离后的初级蛋白质响应中的非键合原子接触传递。此外,目前的结果表明,在蛋白质结构中密集的原子堆积对于响应小分子的缔合和解离的重要性。蛋白质结构的高度紧凑性使结构变化的传播成为可能,为分子机器的设计提供了有用的线索。目前的结果表明,蛋白质结构中密集的原子堆积对于响应小分子的缔合和解离的重要性。蛋白质结构的高度紧凑性使结构变化的传播成为可能,为分子机器的设计提供了有用的线索。目前的结果表明,蛋白质结构中密集的原子堆积对于响应小分子的缔合和解离的重要性。蛋白质结构的高度紧凑性使结构变化的传播成为可能,为分子机器的设计提供了有用的线索。
更新日期:2020-07-02
中文翻译:

非键合原子触点可驱动肌红蛋白中的超快螺旋运动。
小配体的缔合和解离通过蛋白质的结构变化调节蛋白质的功能。这种结构变化会传播很长的距离,并且这种构象在分子功能中起关键作用。但是,人们对传递结构变化的机制了解甚少。在这里,我们显示非结合的原子接触在皮秒级时域中的一氧化碳从肌红蛋白中的血红素基团解离后,在驱动螺旋位移的皮秒级尺度一级结构变化中起着至关重要的作用。目前的时间分辨紫外共振拉曼研究表明,该螺旋位移的幅度在取代Val68时降低,该Val68与野生型肌红蛋白中的血红素接触,但侧链较少(Ala)。我们的发现提供了第一个直接证据,表明结构变化不仅通过共价键,盐桥和氢键传递,而且还通过配体解离后的初级蛋白质响应中的非键合原子接触传递。此外,目前的结果表明,在蛋白质结构中密集的原子堆积对于响应小分子的缔合和解离的重要性。蛋白质结构的高度紧凑性使结构变化的传播成为可能,为分子机器的设计提供了有用的线索。目前的结果表明,蛋白质结构中密集的原子堆积对于响应小分子的缔合和解离的重要性。蛋白质结构的高度紧凑性使结构变化的传播成为可能,为分子机器的设计提供了有用的线索。目前的结果表明,蛋白质结构中密集的原子堆积对于响应小分子的缔合和解离的重要性。蛋白质结构的高度紧凑性使结构变化的传播成为可能,为分子机器的设计提供了有用的线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号