当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
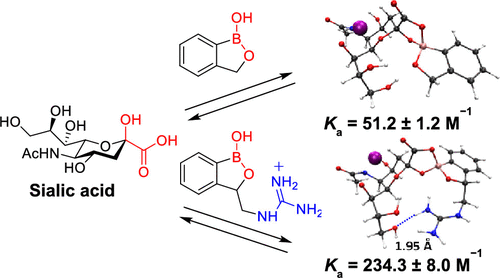
Cooperative Multipoint Recognition of Sialic Acid by Benzoboroxole-Based Receptors Bearing Cationic Hydrogen-Bond Donors.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.joc.0c00173 Alice Di Pasquale 1 , Stefano Tommasone 1 , Lili Xu 2 , Jing Ma 2 , Paula M Mendes 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.joc.0c00173 Alice Di Pasquale 1 , Stefano Tommasone 1 , Lili Xu 2 , Jing Ma 2 , Paula M Mendes 1
Affiliation
|
Sialic acid recognition remains an interesting and challenging target in molecular receptor design. Herein, we report a series of benzoboroxole-based receptors in which cationic hydrogen-bond donors have been introduced and shown to promote multipoint sialic acid recognition. One striking feature revealed by these receptors is that the carboxylate sialic acid residue is the primary binding determinant for recognition by benzoboroxole, in which the presence of charge-reinforced hydrogen bonds results in enhanced selectivity for sialic acid over other carbohydrates and a 4.5-fold increase in affinity. These findings open up wide possibilities for benzoboroxole-based receptors use in life science research, biotechnology, and diagnostics.
中文翻译:

带有阳离子氢键供体的基于苯并硼恶唑的受体对唾液酸的多点协作识别。
唾液酸识别仍然是分子受体设计中一个有趣且具有挑战性的目标。在这里,我们报告了一系列基于苯并硼氧烷的受体,其中引入了阳离子氢键供体,并显示出可促进多点唾液酸识别。这些受体揭示的一个显着特征是,羧酸唾液酸残基是被苯并硼唑识别的主要结合决定因素,其中电荷增强的氢键的存在导致唾液酸对其他碳水化合物的选择性增强,并增加了4.5倍。在亲和力。这些发现为在生命科学研究,生物技术和诊断学中使用基于苯并硼氧烷的受体提供了广泛的可能性。
更新日期:2020-07-02
中文翻译:

带有阳离子氢键供体的基于苯并硼恶唑的受体对唾液酸的多点协作识别。
唾液酸识别仍然是分子受体设计中一个有趣且具有挑战性的目标。在这里,我们报告了一系列基于苯并硼氧烷的受体,其中引入了阳离子氢键供体,并显示出可促进多点唾液酸识别。这些受体揭示的一个显着特征是,羧酸唾液酸残基是被苯并硼唑识别的主要结合决定因素,其中电荷增强的氢键的存在导致唾液酸对其他碳水化合物的选择性增强,并增加了4.5倍。在亲和力。这些发现为在生命科学研究,生物技术和诊断学中使用基于苯并硼氧烷的受体提供了广泛的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号