当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human γS-Crystallin-Copper Binding Helps Buffer against Aggregation Caused by Oxidative Damage.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.biochem.0c00293 Kyle W Roskamp 1 , Sana Azim 2 , Günther Kassier 2 , Brenna Norton-Baker 1, 2 , Marc A Sprague-Piercy 3 , R J Dwyane Miller 2, 4 , Rachel W Martin 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.biochem.0c00293 Kyle W Roskamp 1 , Sana Azim 2 , Günther Kassier 2 , Brenna Norton-Baker 1, 2 , Marc A Sprague-Piercy 3 , R J Dwyane Miller 2, 4 , Rachel W Martin 1, 3
Affiliation
|
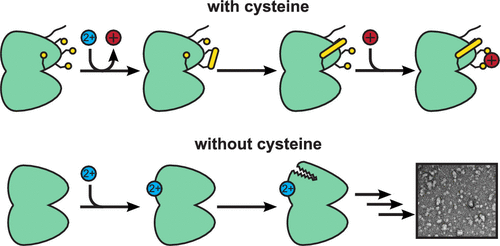
Divalent metal cations can play a role in protein aggregation diseases, including cataract. Here we compare the aggregation of human γS-crystallin, a key structural protein of the eye lens, via mutagenesis, ultraviolet light damage, and the addition of metal ions. All three aggregation pathways result in globular, amorphous-looking structures that do not elongate into fibers. We also investigate the molecular mechanism underlying copper(II)-induced aggregation. This work was motivated by the observation that zinc(II)-induced aggregation of γS-crystallin is driven by intermolecular bridging of solvent-accessible cysteine residues, while in contrast, copper(II)-induced aggregation of this protein is exacerbated by the removal of solvent-accessible cysteines via mutation. Here we find that copper(II)-induced aggregation results from a complex mechanism involving multiple interactions with the protein. The initial protein–metal interactions result in the reduction of Cu(II) to Cu(I) with concomitant oxidation of γS-crystallin. In addition to the intermolecular disulfides that represent a starting point for aggregation, intramolecular disulfides also occur in the cysteine loop, a region of the N-terminal domain that was previously found to mediate the early stages of cataract formation. This previously unobserved ability of γS-crystallin to transfer disulfides intramolecularly suggests that it may serve as an oxidation sink for the lens after glutathione levels have become depleted during aging. γS-Crystallin thus serves as the last line of defense against oxidation in the eye lens, a result that underscores the chemical functionality of this protein, which is generally considered to play a purely structural role.
中文翻译:

人γS-结晶蛋白-铜的结合有助于缓冲液抗氧化损伤引起的聚集。
二价金属阳离子可在蛋白质聚集疾病(包括白内障)中起作用。在这里,我们通过诱变,紫外线损伤和添加金属离子,比较了人眼晶状体的关键结构蛋白-γS-晶状蛋白的聚集。所有这三种聚集途径均导致球形,无定形的结构不会伸长为纤维。我们还研究了铜(II)诱导聚集的分子机制。这项工作的动机是观察到锌(II)诱导的γS-晶状蛋白的聚集是由溶剂可及的半胱氨酸残基的分子间桥接驱动的,而相反,铜(II)诱导的该蛋白质的聚集因去除而加剧突变获得溶剂可及的半胱氨酸。在这里,我们发现铜(II)诱导的聚集是由涉及与蛋白质的多次相互作用的复杂机制导致的。最初的蛋白质-金属相互作用导致γS-晶状体蛋白伴随氧化而将Cu(II)还原为Cu(I)。除了代表聚集起点的分子间二硫化物外,分子内二硫化物也存在于半胱氨酸环中,该半胱氨酸环是先前发现的介导白内障形成早期阶段的N末端结构域区域。以前无法观察到的γS-晶状蛋白分子内转移二硫键的能力表明,在老化过程中谷胱甘肽水平耗尽后,它可能充当晶状体的氧化沉。因此,γS-晶状体蛋白可以作为防止晶状体氧化的最后一道防线,
更新日期:2020-06-30
中文翻译:

人γS-结晶蛋白-铜的结合有助于缓冲液抗氧化损伤引起的聚集。
二价金属阳离子可在蛋白质聚集疾病(包括白内障)中起作用。在这里,我们通过诱变,紫外线损伤和添加金属离子,比较了人眼晶状体的关键结构蛋白-γS-晶状蛋白的聚集。所有这三种聚集途径均导致球形,无定形的结构不会伸长为纤维。我们还研究了铜(II)诱导聚集的分子机制。这项工作的动机是观察到锌(II)诱导的γS-晶状蛋白的聚集是由溶剂可及的半胱氨酸残基的分子间桥接驱动的,而相反,铜(II)诱导的该蛋白质的聚集因去除而加剧突变获得溶剂可及的半胱氨酸。在这里,我们发现铜(II)诱导的聚集是由涉及与蛋白质的多次相互作用的复杂机制导致的。最初的蛋白质-金属相互作用导致γS-晶状体蛋白伴随氧化而将Cu(II)还原为Cu(I)。除了代表聚集起点的分子间二硫化物外,分子内二硫化物也存在于半胱氨酸环中,该半胱氨酸环是先前发现的介导白内障形成早期阶段的N末端结构域区域。以前无法观察到的γS-晶状蛋白分子内转移二硫键的能力表明,在老化过程中谷胱甘肽水平耗尽后,它可能充当晶状体的氧化沉。因此,γS-晶状体蛋白可以作为防止晶状体氧化的最后一道防线,









































 京公网安备 11010802027423号
京公网安备 11010802027423号