当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Incorporation of Sorbed Molybdate during Iron(II)-Induced Transformation of Ferrihydrite and Goethite under Advective Flow Conditions
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-06-08 , DOI: 10.1021/acsearthspacechem.0c00099 Valerie A. Schoepfer 1 , Kaixuan Qin 1 , Jared M. Robertson 2 , Soumya Das 1 , Matthew B. J. Lindsay 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-06-08 , DOI: 10.1021/acsearthspacechem.0c00099 Valerie A. Schoepfer 1 , Kaixuan Qin 1 , Jared M. Robertson 2 , Soumya Das 1 , Matthew B. J. Lindsay 1
Affiliation
|
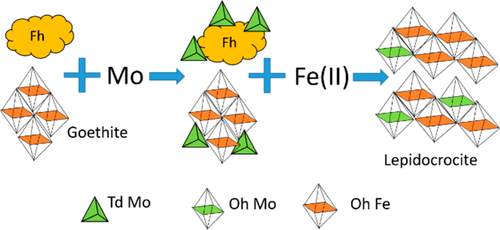
Aqueous Fe(II) can induce recrystallization of ferrihydrite and goethite [α-FeOOH] to their more crystalline or molecularly homogeneous counterparts. Despite common association with these and other Fe(III) (oxyhydr)oxides, relationships between Fe(II)-induced transformation and Mo mobility remain poorly constrained. We conducted laboratory column experiments to examine repartitioning of sorbed Mo during Fe(II)-induced transformation of ferrihydrite and goethite under advective flow conditions. We first pumped (∼0.25 L d–1) artificial groundwater containing 0.1 mM MoO42– and buffered to pH 6.5 through columns packed with ferrihydrite- and goethite-coated sand until >90% Mo breakthrough was observed. Extended X-ray absorption fine structure (EXAFS) spectroscopy shows that initial Mo attenuation resulted from inner sphere complexation of MoO4 tetrahedra at ferrihydrite and goethite surfaces. We then pumped Mo-free anoxic artificial groundwater containing 0.2 mM or 2.0 mM Fe(II) through the columns until effluent Mo concentrations remained <0.005 mM. Raman spectroscopy shows that Fe(II) introduction induced transformation of both ferrihydrite and goethite to lepidocrocite. Additionally, Fe(II) introduction mobilized 4–34% of sorbed Mo and total mass release was greater for (i) ferrihydrite compared to goethite columns and (ii) low Fe(II) compared to high Fe(II) influent. Effluent pH decreased to ∼5.8 for columns receiving the high Fe(II) influent and returned to pH 6.5 after 5–10 pore volumes. EXAFS spectroscopy indicates that structural incorporation of MoO6 octahedra into neoformed phases contributes to Mo retention during Fe(II)-induced transformation. Our results offer new insight into Mo repartitioning during Fe(II)-induced transformation of Fe(III) (oxyhydr)oxides and, more generally, controls on Mo mobility in geohydrologic systems.
中文翻译:

在正向流动条件下铁(II)引起的水铁矿和针铁矿转变过程中吸附的钼酸盐的结构结合
Fe(II)水溶液可诱导水铁矿和针铁矿[α-FeOOH]重结晶为结晶度更高或分子均质的对应物。尽管与这些和其他Fe(III)(羟基)氧化物有共同的关联,但Fe(II)诱导的转化与Mo迁移率之间的关系仍然受约束较弱。我们进行了实验室柱实验,以研究在平流条件下Fe(II)引起的水铁矿和针铁矿转变过程中吸附的Mo的重新分配。我们首先抽水(〜0.25 L d –1)含有0.1 mM MoO 4 2–并通过装有三水铁矿和针铁矿覆盖的砂子的柱子将其缓冲至pH 6.5,直到观察到Mo突破> 90%。扩展的X射线吸收精细结构(EXAFS)光谱表明,初始Mo衰减是由MoO 4的内球络合引起的在水铁矿和针铁矿表面的四面体。然后,我们将含有0.2 mM或2.0 mM Fe(II)的无Mo缺氧人工地下水泵入色谱柱,直到流出的Mo浓度保持<0.005 mM。拉曼光谱法表明,Fe(II)的引入可引起水铁矿和针铁矿均转变为纤铁矿。另外,Fe(II)的引入动员了4%到34%的吸附Mo,(i)水铁矿比针铁矿柱和(ii)低Fe(II)的铁(II)进料的总质量释放更大。对于接受高Fe(II)进水的色谱柱,出水pH降至5.8,并在5-10孔体积后回到pH 6.5。EXAFS光谱表明MoO 6的结构结合八面体进入新形成的相有助于在Fe(II)诱导的转变过程中保留Mo。我们的结果提供了新的见解,了解了在Fe(II)诱导的Fe(III)(羟基)氧化物转化过程中Mo的重新分配,以及更广泛地控制了地热系统中Mo的迁移率。
更新日期:2020-07-16
中文翻译:

在正向流动条件下铁(II)引起的水铁矿和针铁矿转变过程中吸附的钼酸盐的结构结合
Fe(II)水溶液可诱导水铁矿和针铁矿[α-FeOOH]重结晶为结晶度更高或分子均质的对应物。尽管与这些和其他Fe(III)(羟基)氧化物有共同的关联,但Fe(II)诱导的转化与Mo迁移率之间的关系仍然受约束较弱。我们进行了实验室柱实验,以研究在平流条件下Fe(II)引起的水铁矿和针铁矿转变过程中吸附的Mo的重新分配。我们首先抽水(〜0.25 L d –1)含有0.1 mM MoO 4 2–并通过装有三水铁矿和针铁矿覆盖的砂子的柱子将其缓冲至pH 6.5,直到观察到Mo突破> 90%。扩展的X射线吸收精细结构(EXAFS)光谱表明,初始Mo衰减是由MoO 4的内球络合引起的在水铁矿和针铁矿表面的四面体。然后,我们将含有0.2 mM或2.0 mM Fe(II)的无Mo缺氧人工地下水泵入色谱柱,直到流出的Mo浓度保持<0.005 mM。拉曼光谱法表明,Fe(II)的引入可引起水铁矿和针铁矿均转变为纤铁矿。另外,Fe(II)的引入动员了4%到34%的吸附Mo,(i)水铁矿比针铁矿柱和(ii)低Fe(II)的铁(II)进料的总质量释放更大。对于接受高Fe(II)进水的色谱柱,出水pH降至5.8,并在5-10孔体积后回到pH 6.5。EXAFS光谱表明MoO 6的结构结合八面体进入新形成的相有助于在Fe(II)诱导的转变过程中保留Mo。我们的结果提供了新的见解,了解了在Fe(II)诱导的Fe(III)(羟基)氧化物转化过程中Mo的重新分配,以及更广泛地控制了地热系统中Mo的迁移率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号