当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expression, purification, crystallization and X-ray diffraction studies of a novel root-induced secreted protein from Trichoderma virens.
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-06-08 , DOI: 10.1107/s2053230x20007025 Ravindra Bansal 1 , Hiral U Mistry 2 , Prasun K Mukherjee 1 , Gagan D Gupta 2
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-06-08 , DOI: 10.1107/s2053230x20007025 Ravindra Bansal 1 , Hiral U Mistry 2 , Prasun K Mukherjee 1 , Gagan D Gupta 2
Affiliation
|
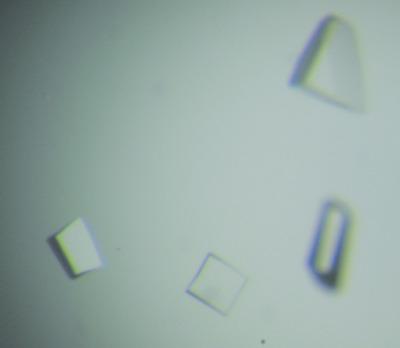
Small secreted cysteine‐rich proteins (SSCPs) from fungi play an important role in fungi–host interactions. The plant‐beneficial fungi Trichoderma spp. are in use worldwide as biocontrol agents and protect the host plant from soil‐borne as well as foliar pathogens. Recently, a novel SSCP, Tsp1, has been identified in the secreted protein pool of T. virens and is overinduced upon its interaction with the roots of the maize plant. The protein was observed to be well conserved in the Ascomycota division of fungi, and its homologs are present in many plant‐pathogenic fungi such as Fusarium oxysporum and Magnaporthe oryzae. However, none of these homologs have yet been characterized. Recombinant Tsp1 protein has been expressed and purified using an Escherichia coli expression system. The protein, with four conserved cysteines, forms a dimer in solution as observed by size‐exclusion chromatography. The dimerization, however, does not involve disulfide bonds. Circular‐dichroism data suggested that the protein has a β‐strand‐rich secondary structure that matched well with the secondary structure predicted using bioinformatics methods. The protein was crystallized using sodium malonate as a precipitant. The crystals diffracted X‐rays to 1.7 Å resolution and belonged to the orthorhombic space group P212121 (Rmeas = 5.4%), with unit‐cell parameters a = 46.3, b = 67.0, c = 173.2 Å. The Matthews coefficient (VM) of the crystal is 2.32 Å3 Da−1, which corresponds to nearly 47% solvent content with four subunits of Tsp1 protein in the asymmetric unit. This is the first report of the structural study of any homolog of the novel Tsp1 protein. These structural studies will help in understanding the classification and function of the protein.
中文翻译:

绿色木霉新型根诱导分泌蛋白的表达、纯化、结晶和 X 射线衍射研究。
真菌分泌的富含半胱氨酸的小蛋白(SSCP)在真菌与宿主的相互作用中发挥着重要作用。对植物有益的真菌木霉属。在世界范围内用作生物防治剂,保护宿主植物免受土传和叶面病原体的侵害。最近,在T. virens的分泌蛋白库中发现了一种新的 SSCP,Tsp1,并且在与玉米植物根部相互作用时被过度诱导。据观察,该蛋白质在真菌的子囊菌门中高度保守,其同源物存在于许多植物病原真菌中,例如尖孢镰刀菌和稻瘟病菌。然而,这些同系物尚未被表征。重组 Tsp1 蛋白已使用大肠杆菌表达系统进行表达和纯化。通过尺寸排阻色谱法观察到,该蛋白质具有四个保守的半胱氨酸,在溶液中形成二聚体。然而,二聚化不涉及二硫键。圆二色性数据表明该蛋白质具有富含β链的二级结构,与使用生物信息学方法预测的二级结构非常匹配。使用丙二酸钠作为沉淀剂使蛋白质结晶。该晶体的 X 射线衍射分辨率为 1.7 Å,属于斜方空间群P 2 1 2 1 2 1 ( R meas = 5.4%),晶胞参数a = 46.3、b = 67.0、c = 173.2 Å。晶体的马修斯系数( VM )为2.32 Å 3 Da -1,对应于不对称单元中Tsp1蛋白的四个亚基的近47%溶剂含量。这是对新型 Tsp1 蛋白同源物的结构研究的第一份报告。这些结构研究将有助于理解蛋白质的分类和功能。
更新日期:2020-06-08
中文翻译:

绿色木霉新型根诱导分泌蛋白的表达、纯化、结晶和 X 射线衍射研究。
真菌分泌的富含半胱氨酸的小蛋白(SSCP)在真菌与宿主的相互作用中发挥着重要作用。对植物有益的真菌木霉属。在世界范围内用作生物防治剂,保护宿主植物免受土传和叶面病原体的侵害。最近,在T. virens的分泌蛋白库中发现了一种新的 SSCP,Tsp1,并且在与玉米植物根部相互作用时被过度诱导。据观察,该蛋白质在真菌的子囊菌门中高度保守,其同源物存在于许多植物病原真菌中,例如尖孢镰刀菌和稻瘟病菌。然而,这些同系物尚未被表征。重组 Tsp1 蛋白已使用大肠杆菌表达系统进行表达和纯化。通过尺寸排阻色谱法观察到,该蛋白质具有四个保守的半胱氨酸,在溶液中形成二聚体。然而,二聚化不涉及二硫键。圆二色性数据表明该蛋白质具有富含β链的二级结构,与使用生物信息学方法预测的二级结构非常匹配。使用丙二酸钠作为沉淀剂使蛋白质结晶。该晶体的 X 射线衍射分辨率为 1.7 Å,属于斜方空间群P 2 1 2 1 2 1 ( R meas = 5.4%),晶胞参数a = 46.3、b = 67.0、c = 173.2 Å。晶体的马修斯系数( VM )为2.32 Å 3 Da -1,对应于不对称单元中Tsp1蛋白的四个亚基的近47%溶剂含量。这是对新型 Tsp1 蛋白同源物的结构研究的第一份报告。这些结构研究将有助于理解蛋白质的分类和功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号