当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiomeric NMR Signal Separation Mechanism and Prediction of Separation Behavior for a Praseodymium (III) Complex with (S ,S )-Ethylenediamine-N ,N ’-Disuccinate
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-07-08 , DOI: 10.1002/mrc.5062 Sen-Ichi Aizawa 1 , Masaru Okano 2
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-07-08 , DOI: 10.1002/mrc.5062 Sen-Ichi Aizawa 1 , Masaru Okano 2
Affiliation
|
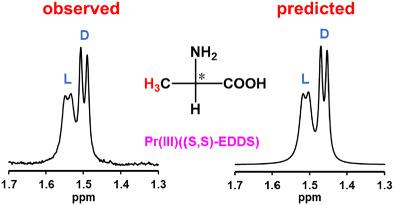
Since choice of chiral NMR shift reagents and concentration conditions have been made empirically by trials and errors for chiral NMR analyses, the prediction of NMR signal separation behavior is an urgent issue. In this study, the separation of enantiomeric and enantiotopic 1 H and 13 C NMR signals for α-amino acids and tartaric acid were performed by using the praseodymium (III) complex with (S,S)-ethylenediamine-N,N'-disuccinate ((S,S)-EDDS). All the present D-amino acids exhibited larger downfield shift of their α-protons and α-carbons compared with those for the corresponding L-amino acids in common. This regularity is applicable to absolute configurational assignment and determination of optical purity of amino acids. The chemical shifts of β-protons of D- and L-alanine fully bound with the Pr (III)((S,S)-EDDS) complex (δb s) and the adduct formation constants of both enantiomers (Ks) were obtained by dependences of the observed downfield shifts of the β-protons on the total concentrations of the respective enantiomers in the presence of a constant concentration of the Pr (III) complex. The difference in the K values was found to be predominant determining factor for the enantiomeric signal separation. The chemical shifts of both enantiomers (δs) and the enantiomeric signal separations (Δδs) under given conditions could be calculated from the δb and K values. Furthermore, prediction of the signal separation behavior was enabled by using the calculated δ values and the signal broadening obtained by dependences of the half-height widths of the observed signals on the bound/free substrate concentration ratios for the respective enantiomers.
中文翻译:

(S ,S )-乙二胺-N ,N'-二琥珀酸镨 (III) 配合物的对映体 NMR 信号分离机制和分离行为预测
由于手性 NMR 位移试剂和浓度条件的选择是通过手性 NMR 分析的试验和错误凭经验做出的,因此 NMR 信号分离行为的预测是一个紧迫的问题。在本研究中,使用镨 (III) 与 (S,S)-乙二胺-N,N'-二琥珀酸酯的对映体和对映体 1 H 和 13 C NMR 信号的分离((S,S)-EDDS)。与相应的 L-氨基酸相比,所有现有的 D-氨基酸都表现出更大的 α-质子和 α-碳的低场位移。这种规律适用于氨基酸的绝对构型分配和光学纯度的测定。D-和L-丙氨酸的β-质子的化学位移完全与Pr (III)((S, S)-EDDS) 复合物 (δb s) 和两种对映体 (Ks) 的加合物形成常数是通过观察到的 β-质子的低场位移对各对映体在恒定浓度存在下的总浓度的依赖性而获得的Pr (III) 络合物。发现 K 值的差异是对映体信号分离的主要决定因素。可以从 δb 和 K 值计算给定条件下对映体 (δs) 和对映体信号分离 (Δδs) 的化学位移。此外,通过使用计算的 δ 值和信号展宽,预测信号分离行为是可行的,该信号展宽由观察到的信号的半高宽度对各个对映异构体的结合/游离底物浓度比的依赖性获得。
更新日期:2020-07-08
中文翻译:

(S ,S )-乙二胺-N ,N'-二琥珀酸镨 (III) 配合物的对映体 NMR 信号分离机制和分离行为预测
由于手性 NMR 位移试剂和浓度条件的选择是通过手性 NMR 分析的试验和错误凭经验做出的,因此 NMR 信号分离行为的预测是一个紧迫的问题。在本研究中,使用镨 (III) 与 (S,S)-乙二胺-N,N'-二琥珀酸酯的对映体和对映体 1 H 和 13 C NMR 信号的分离((S,S)-EDDS)。与相应的 L-氨基酸相比,所有现有的 D-氨基酸都表现出更大的 α-质子和 α-碳的低场位移。这种规律适用于氨基酸的绝对构型分配和光学纯度的测定。D-和L-丙氨酸的β-质子的化学位移完全与Pr (III)((S, S)-EDDS) 复合物 (δb s) 和两种对映体 (Ks) 的加合物形成常数是通过观察到的 β-质子的低场位移对各对映体在恒定浓度存在下的总浓度的依赖性而获得的Pr (III) 络合物。发现 K 值的差异是对映体信号分离的主要决定因素。可以从 δb 和 K 值计算给定条件下对映体 (δs) 和对映体信号分离 (Δδs) 的化学位移。此外,通过使用计算的 δ 值和信号展宽,预测信号分离行为是可行的,该信号展宽由观察到的信号的半高宽度对各个对映异构体的结合/游离底物浓度比的依赖性获得。











































 京公网安备 11010802027423号
京公网安备 11010802027423号