Medicinal Chemistry ( IF 1.9 ) Pub Date : 2020-05-31 , DOI: 10.2174/1573406415666190430125547 Rong Y. Han 1 , Yu Ge 1 , Ling Zhang 1 , Qing M. Wang 1
|
Background: Protein tyrosine phosphatases 1B are considered to be a desirable validated target for therapeutic development of type II diabetes and obesity.
Methods: A new series of imidazolyl flavonoids as potential protein tyrosine phosphatase inhibitors were synthesized and evaluated.
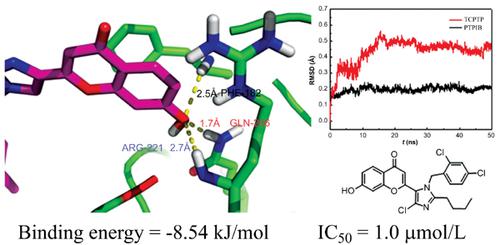
Results: Bioactive results indicated that some synthesized compounds exhibited potent protein phosphatase 1B (PTP1B) inhibitory activities at the micromolar range. Especially, compound 8b showed the best inhibitory activity (IC50=1.0 µM) with 15-fold selectivity for PTP1B over the closely related T-cell protein tyrosine phosphatase (TCPTP). Cell viability assays indicated that 8b is cell permeable with lower cytotoxicity. Molecular modeling and dynamics studies revealed the reason for selectivity of PTP1B over TCPTP. Quantum chemical studies were carried out on these compounds to understand the structural features essential for activity.
Conclusion: Compound 8b should be a potential selective PTP1B inhibitor.
中文翻译:

新型咪唑基类黄酮作为强效和选择性蛋白酪氨酸磷酸酶抑制剂的设计和生物学评价
背景:蛋白酪氨酸磷酸酶1B被认为是II型糖尿病和肥胖症治疗发展的理想验证靶标。
方法:合成并评估了一系列新的咪唑基类黄酮作为潜在的蛋白酪氨酸磷酸酶抑制剂。
结果:生物活性结果表明,某些合成的化合物在微摩尔范围内表现出有效的蛋白磷酸酶1B(PTP1B)抑制活性。尤其是,化合物8b表现出最好的抑制活性(IC50 = 1.0 µM),其PTP1B的选择性是紧密相关的T细胞蛋白酪氨酸磷酸酶(TCPTP)的15倍。细胞活力测定表明8b是细胞可渗透的,具有较低的细胞毒性。分子建模和动力学研究揭示了PTP1B相对于TCPTP选择性的原因。对这些化合物进行了量子化学研究,以了解活性必不可少的结构特征。
结论:化合物8b应该是潜在的选择性PTP1B抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号