Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-05-01 , DOI: 10.2174/1570180816666190618111023 Mehlika Dilek Altıntop 1
|
Background: Pyrazolines, electron-rich nitrogen carriers, are of great importance due to their potential applications for the treatment of many diseases including inflammation, infectious diseases and neurodegenerative disorders.
Objectives: The purpose of this work was to synthesize new pyrazoline derivatives and evaluate their anticholinesterase effects.
Methods: 1-Aryl-5-[4-(piperidin-1-yl)phenyl]-3-(3,4-dimethoxyphenyl)-4,5-dihydro-1H-pyrazoles (1-7) were synthesized via the treatment of 1-(3,4-dimethoxyphenyl)-3-[4-(piperidin-1-yl)phenyl]prop-2- en-1-one with arylhydrazine hydrochloride derivatives in acetic acid, whereas 1-aryl-5-[4- (morpholin-4-yl)phenyl]-3-(3,4-dimethoxyphenyl)-4,5-dihydro-1H-pyrazoles (8-14) were obtained by the treatment of 1-(3,4-dimethoxyphenyl)-3-[4-(morpholin-4-yl)phenyl]prop-2-en-1-one with arylhydrazine hydrochloride derivatives in acetic acid. Their inhibitory effects on acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) were determined using a modification of Ellman’s spectrophotometric method. In silico docking and Absorption, Distribution, Metabolism and Excretion (ADME) studies were performed using Schrödinger’s Maestro molecular modeling package.
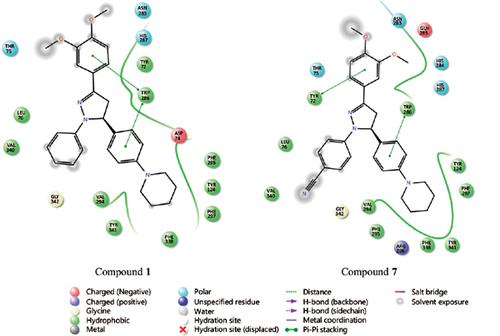
Results: In general, piperidine derivatives were found to be more effective than morpholine derivatives on cholinesterases (ChEs). 1-Phenyl-5-[4-(piperidin-1-yl)phenyl]-3-(3,4-dimethoxyphenyl)- 4,5-dihydro-1H-pyrazole (1) and 1-(4-cyanophenyl)-5-[4-(piperidin-1-yl)phenyl]-3-(3,4- dimethoxyphenyl)-4,5-dihydro-1H-pyrazole (7) were identified as the most effective AChE inhibitors in this series with 40.92% and 38.98%, respectively. Compounds 1 and 7 were docked into the active site of human AChE (PDB code: 4EY7). Both the compounds were found to be capable of forming π-π stacking interactions with Trp286. Based on in silico ADME studies, these compounds are expected to have reasonable oral bioavailability.
Conclusion: In the view of this work, the structural modification of the identified agents is going on for the generation of new anticholinesterase agents with enhanced efficacy.
中文翻译:

一系列吡唑啉类新抗胆碱酯酶药物的合成,体外和计算机评价
背景:吡唑啉是富含电子的氮载体,由于其在治疗多种疾病(包括炎症,传染性疾病和神经退行性疾病)中的潜在应用而具有重要意义。
目的:这项工作的目的是合成新的吡唑啉衍生物并评估其抗胆碱酯酶的作用。
方法:通过处理合成了1-芳基-5- [4-(哌啶-1-基)苯基] -3-(3,4-二甲氧基苯基)-4,5-二氢-1H-吡唑(1-7)。 1-(3,4-二甲氧基苯基)-3- [4-(哌啶-1-基)苯基] prop-2-en-1-one与芳基肼盐酸盐衍生物在乙酸中的合成,而1-芳基-5- [通过处理1-(3,4-二甲氧基苯基)获得4-(吗啉-4-基)苯基] -3-(3,4-二甲氧基苯基)-4,5-二氢-1H-吡唑(8-14)。 )-3- [4-(吗啉-4-基)苯基]丙-2-烯-1-酮与乙酸芳基肼的衍生物。使用改良的Ellman分光光度法确定了它们对乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BuChE)的抑制作用。在计算机对接和吸收,分布,代谢和排泄(ADME)研究中,使用了Schrödinger的Maestro分子建模软件包。
结果:通常,发现哌啶衍生物在胆碱酯酶(ChEs)上比吗啉衍生物更有效。1-苯基-5- [4-(哌啶-1-基)苯基] -3-(3,4-二甲氧基苯基)-4,5-二氢-1H-吡唑(1)和1-(4-氰基苯基)- 5- [4-(哌啶-1-基)苯基] -3-(3,4-二甲氧基苯基)-4,5-二氢-1H-吡唑(7)被鉴定为该系列中最有效的AChE抑制剂,占40.92 %和38.98%。将化合物1和7插入人类AChE的活性位点(PDB代码:4EY7)。发现这两种化合物都能够与Trp286形成π-π堆积相互作用。根据计算机模拟ADME研究,预计这些化合物具有合理的口服生物利用度。
结论:鉴于这项工作,对所鉴定药物的结构进行了修饰,以产生具有增强功效的新型抗胆碱酯酶药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号