Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-05-01 , DOI: 10.2174/1570180816666181108115510 Sara Azimi 1 , Omidreza Firuzi 2 , Aida Iraji 2 , Afsaneh Zonouzi 1 , Mahsima Khoshneviszadeh 2 , Mohammad Mahdavi 3 , Najmeh Edraki 2
|
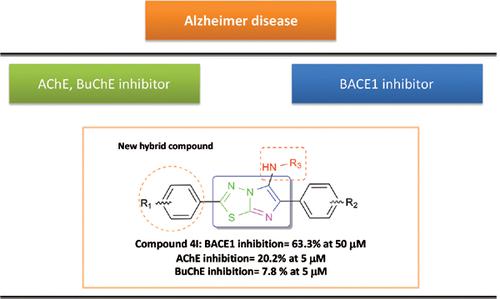
Background: Considering that AD is multifactorial in nature, novel series of imidazo [2,1-b][1,3,4] thiadiazole derivatives were designed to address the basic factors responsible for the disease.
Methods: These compounds were investigated as inhibitors of beta-site APP cleaving enzyme 1, acetylcholinesterase and butyryl cholinesterase.
Results: The BACE1 inhibitory results indicated that nitro phenyl substituted derivatives of imidazo [2,1-b][1,3,4] thiadiazole scaffold (R2 = m-NO2) demonstrated superior BACE1 inhibitory activity compared to other substituted moieties. In the BuChE assay, compounds 4h and 4l carrying meta NO2 at R2 of phenyl ring turned out to be potent inhibitors.
Conclusion: In conclusion, these novel synthesized derivatives seem to be promising anti-Alzheimer agents.
中文翻译:

新型咪唑并[2,1-B] [1,3,4]噻二唑的合成及其体外生物活性评价
背景:考虑到AD本质上是多因素的,因此设计了一系列新的咪唑并[2,1-b] [1,3,4]噻二唑衍生物来解决造成该疾病的基本因素。
方法:研究了这些化合物作为β-位APP裂解酶1,乙酰胆碱酯酶和丁酰胆碱酯酶的抑制剂。
结果:BACE1抑制结果表明,咪唑并[2,1-b] [1,3,4]噻二唑支架(R2 = m-NO2)的硝基苯基取代衍生物比其他取代部分具有更好的BACE1抑制活性。在BuChE测定中,在苯环的R 2上带有间NO 2的化合物4h和4l被证明是有效的抑制剂。
结论:总之,这些新颖的合成衍生物似乎是有希望的抗阿尔茨海默病药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号