Current Bioinformatics ( IF 2.4 ) Pub Date : 2020-02-29 , DOI: 10.2174/1574893614666191212112026 Avirup Ghosh 1 , Hong Yan 1
|
Background: Mutations in a protein called the Epidermal Growth Factor Receptor (EGFR) can cause Non-Small Cell Lung Cancer (NSCLC), which is the most common form of lung cancer. Many NSCLC cases arise from the L858R mutation, where Leucine (L) is replaced by arginine (R) at the 858th position in the EGFR, and that is also recognized as an exon 21 substitution. Moreover, half of the EKFR-mutated lung cancer patients develop acquired resistance to the first-generation EGFR-TKIs due to another mutation T790M.
Objective: In this research work, a novel method is used to investigate the possible reason for the EGFR mutation to takes place in the specific 858th and 790th position, and also, we evaluated the hydrogen bonds to measure the overall stability of different structures.
Methods: We performed the molecular dynamics simulation and used Amber tool to achieve our primary objectives and later we use CPPTRAJ to analyze other changes in the hydrogen bonds for different mutational structures of EGFR.
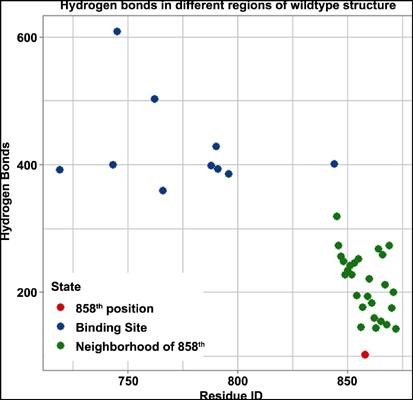
Results: First, we investigated the hydrogen bonds in different positions in the EGFR kinase domain and estimated why the first stage mutation (L858R) and resistance mutation (L858R/T790M) take place in the 858th and 790th position respectively. We found the hydrogen bond counts in the 858th and 790th position is lesser than the neighborhood positions and that yields to achieve a least stability in that position.
Conclusion: Our method represents an important contribution to molecular dynamics analysis for NSCLC studies. The results obtained from this study provide a useful insight into the NSCLC drug resistance.
中文翻译:

非小细胞肺癌相关EGFR关键部位的稳定性分析
背景:称为表皮生长因子受体(EGFR)的蛋白质中的突变会引起非小细胞肺癌(NSCLC),这是肺癌的最常见形式。许多NSCLC病例是由L858R突变引起的,其中亮氨酸(L)在EGFR的858位被精氨酸(R)取代,并且也被认为是外显子21取代。此外,由于另一种突变T790M,一半EKFR突变的肺癌患者对第一代EGFR-TKI产生了获得性耐药。
目的:在这项研究工作中,使用一种新方法来研究在第858位和第790位特定位置发生EGFR突变的可能原因,并且我们还评估了氢键以测量不同结构的整体稳定性。
方法:我们进行了分子动力学模拟,并使用Amber工具达到了主要目标,随后我们使用CPPTRAJ分析了EGFR不同突变结构的氢键的其他变化。
结果:首先,我们研究了EGFR激酶结构域中不同位置的氢键,并估计了为什么第一阶段突变(L858R)和抗性突变(L858R / T790M)分别发生在第858位和790位。我们发现,第858位和第790位的氢键数小于邻位,且屈服度在该位置达到最低稳定性。
结论:我们的方法代表了NSCLC研究分子动力学分析的重要贡献。这项研究获得的结果为NSCLC耐药性提供了有用的见识。









































 京公网安备 11010802027423号
京公网安备 11010802027423号