Current Analytical Chemistry ( IF 1.7 ) Pub Date : 2020-05-31 , DOI: 10.2174/1573411014666180704114202 Matjaž Finšgar 1 , Klodian Xhanari 1 , Helena O. Ćurković 2
|
Background: Cyclic voltammetry is widely employed in electroanalytical studies because it provides fast information about the redox potentials of the electroactive species and the influence of the medium on the redox processes. Azole compounds have been found to be effective corrosion inhibitors for copper in chloride-containing solutions. The aim of this work was to investigate in detail the influence of the addition of various azole compounds on the oxidation mechanism of copper in chloride-containing solutions, using cyclic voltammetry.
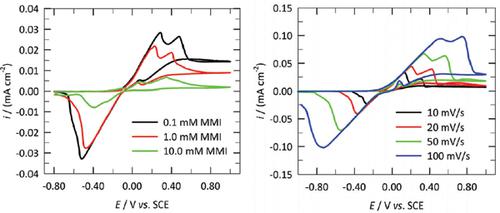
Methods: The influence of thirteen azole compounds, at three different concentrations on the electrochemical/ chemical reactions of pure copper immersed in 3 wt.% NaCl solution was studied using cyclic voltammetry at different scan rates. The change of the peak current and potential with the scan rate were investigated. The possible linearity was compared with the theoretically derived mechanism. The possible reaction mechanisms were discussed based on the linearity of these parameters (peak current and potential) with the scan rate compared to theoretically derived models.
Results: Both the peak current and peak potential of the copper samples immersed in chloridecontaining solutions with additions of the majority of azole compounds showed linearity with the square root of the scan rate, suggesting that copper follows the Müller-Calandra passivation model. The same behavior was also found for copper in chloride-containing solutions without additions of azole compounds. A linear variation of the peak potential with the natural logarithm of the scan rate and linear variation of the peak potential with the square root of the scan rate was observed for the copper samples immersed in chloride-containing solutions with the addition of 10 mM of 2-mercapto-1- methylimidazole, imidazole, or 2-aminobenzimidazole. This suggests that copper follows irreversible redox reactions under a diffusion controlled process. No other linear relations of the peak current and peak potential with the scan rate were found.
Conclusion: Copper oxidation in chloride-containing solutions is controlled by passivation (following the Müller-Calandra passivation model) upon the addition of the majority of the selected azoles. In the minority of cases, irreversible redox reactions that follow a diffusion-controlled process were identified. None of the systems followed an adsorption-controlled process. Moreover, none of the tested systems underwent reversible redox reactions that followed a diffusion controlled process.
中文翻译:

循环伏安法作为电分析工具,用于分析铜在含不同腈化合物的氯化物溶液中的反应机理
背景:循环伏安法广泛用于电分析研究中,因为它提供了有关电活性物质的氧化还原电势以及介质对氧化还原过程的影响的快速信息。已经发现,在含氯化物的溶液中,唑化合物是铜的有效腐蚀抑制剂。这项工作的目的是使用循环伏安法,详细研究添加各种唑化合物对含氯溶液中铜的氧化机理的影响。
方法:使用循环伏安法在不同扫描速率下研究了三种不同浓度的13种唑化合物对纯铜浸入3%重量的NaCl溶液中的电化学/化学反应的影响。研究了峰值电流和电位随扫描速率的变化。将可能的线性与理论推导的机制进行了比较。与理论得出的模型相比,基于这些参数(峰值电流和电势)与扫描速率的线性关系,讨论了可能的反应机理。
结果:浸入含氯溶液中并添加了大多数唑化合物的铜样品的峰值电流和峰值电势均与扫描速率的平方根呈线性关系,这表明铜遵循了Müller-Calandra钝化模型。对于不添加唑化合物的含氯化物溶液中的铜,也发现了相同的行为。对于浸入含氯化物溶液中并添加10 mM 2的铜样品,观察到峰电位随扫描速率的自然对数线性变化,峰电位随扫描速率的平方根线性变化。 -巯基-1-甲基咪唑,咪唑或2-氨基苯并咪唑。这表明铜在扩散控制的过程中会发生不可逆的氧化还原反应。
结论:在添加大多数选定的唑类的情况下,通过钝化(遵循Müller-Calandra钝化模型)可控制含氯化物溶液中的铜氧化。在少数情况下,识别出遵循扩散控制过程的不可逆氧化还原反应。没有一个系统遵循吸附控制的过程。而且,没有一个测试过的系统在扩散控制的过程中发生可逆的氧化还原反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号