当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Large-Scale 3D Optical Mapping and Quantitative Analysis of Nanoparticle Distribution in Tumor Vascular Microenvironment.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-06-06 , DOI: 10.1021/acs.bioconjchem.0c00263 Dong-Jun Koo 1, 2 , Jinahn Choi 3 , Minchul Ahn 3, 4 , Benjamin H Ahn 1, 3 , Dal-Hee Min 3, 4 , Sung-Yon Kim 1, 2, 3
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-06-06 , DOI: 10.1021/acs.bioconjchem.0c00263 Dong-Jun Koo 1, 2 , Jinahn Choi 3 , Minchul Ahn 3, 4 , Benjamin H Ahn 1, 3 , Dal-Hee Min 3, 4 , Sung-Yon Kim 1, 2, 3
Affiliation
|
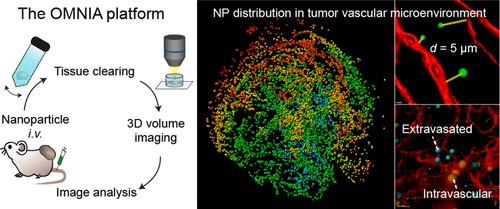
Nanoparticles (NPs) are a promising carrier for cancer therapeutics. Systemically administered NPs are transported to tumor tissues via the bloodstream, extravasated from microvessels, and delivered to cancer cells. The distribution of NPs in the tumor vascular microenvironment critically determines the therapeutic efficacy of NP-delivered drugs, but its precise assessment in 3D across a large volume remains challenging. Here, an analytical platform—termed OMNIA (for Optical Mapping of Nanoparticles and Image Analysis)—integrating tissue clearing, high-resolution optical imaging, and semiautomated image analysis is presented, which enables accurate, unbiased, and quantitative analysis of the distribution of NPs in relation to the vasculature across a large 3D volume. Application of OMNIA to tumor tissues revealed higher accumulation and more efficient extravasation of NPs in the tumor periphery than the core. Time-course analysis demonstrated that the accumulation of NPs in tumor peaked at 24 h after injection, but the relative distribution of NPs from the vasculature remained remarkably stable over time. Comparisons between 45- and 200-nm-sized NPs showed a lower accumulation of smaller NPs in tumors relative to the liver, yet better vessel permeation. Together, our results demonstrate that OMNIA facilitates precise and reliable evaluation of NP biodistribution, and mechanistic investigations on NP delivery to tumor tissues.
中文翻译:

肿瘤血管微环境中纳米颗粒分布的大规模3D光学映射和定量分析。
纳米颗粒(NPs)是用于癌症治疗的有希望的载体。全身给药的NPs通过血流被运输到肿瘤组织,从微血管中渗出并被运输到癌细胞。NPs在肿瘤血管微环境中的分布决定性地决定了NP递送药物的治疗效果,但是在大体积中进行3D精确评估仍然具有挑战性。在这里,介绍了一个称为OMNIA(用于纳米粒子的光学作图和图像分析)的分析平台,该平台集成了组织清除,高分辨率光学成像和半自动图像分析功能,从而可以对NP的分布进行准确,无偏且定量的分析与大3D体积中的脉管系统有关。OMNIA在肿瘤组织上的应用显示,与核心相比,NP外围在肿瘤外围的积累和释放效率更高。时程分析表明,NPs在肿瘤中的积累在注射后24 h达到峰值,但随着时间的推移,来自脉管系统的NPs相对分布仍保持明显稳定。比较45纳米和200纳米大小的NPs,相对于肝脏,较小的NPs在肿瘤中的积累较低,但血管渗透性更好。在一起,我们的结果表明,OMNIA有助于精确和可靠地评估NP生物分布,以及将NP递送至肿瘤组织的机制研究。但是随着时间的流逝,来自脉管系统的NP的相对分布仍然非常稳定。比较45纳米和200纳米大小的NPs,相对于肝脏,较小的NPs在肿瘤中的积累较低,但血管渗透性更好。在一起,我们的结果表明,OMNIA有助于精确和可靠地评估NP生物分布,以及将NP输送到肿瘤组织的机制研究。但是随着时间的流逝,来自脉管系统的NP的相对分布仍然非常稳定。比较45纳米和200纳米大小的NPs,相对于肝脏,较小的NPs在肿瘤中的积累较低,但血管渗透性更好。在一起,我们的结果表明,OMNIA有助于精确和可靠地评估NP生物分布,以及将NP递送至肿瘤组织的机制研究。
更新日期:2020-07-15
中文翻译:

肿瘤血管微环境中纳米颗粒分布的大规模3D光学映射和定量分析。
纳米颗粒(NPs)是用于癌症治疗的有希望的载体。全身给药的NPs通过血流被运输到肿瘤组织,从微血管中渗出并被运输到癌细胞。NPs在肿瘤血管微环境中的分布决定性地决定了NP递送药物的治疗效果,但是在大体积中进行3D精确评估仍然具有挑战性。在这里,介绍了一个称为OMNIA(用于纳米粒子的光学作图和图像分析)的分析平台,该平台集成了组织清除,高分辨率光学成像和半自动图像分析功能,从而可以对NP的分布进行准确,无偏且定量的分析与大3D体积中的脉管系统有关。OMNIA在肿瘤组织上的应用显示,与核心相比,NP外围在肿瘤外围的积累和释放效率更高。时程分析表明,NPs在肿瘤中的积累在注射后24 h达到峰值,但随着时间的推移,来自脉管系统的NPs相对分布仍保持明显稳定。比较45纳米和200纳米大小的NPs,相对于肝脏,较小的NPs在肿瘤中的积累较低,但血管渗透性更好。在一起,我们的结果表明,OMNIA有助于精确和可靠地评估NP生物分布,以及将NP递送至肿瘤组织的机制研究。但是随着时间的流逝,来自脉管系统的NP的相对分布仍然非常稳定。比较45纳米和200纳米大小的NPs,相对于肝脏,较小的NPs在肿瘤中的积累较低,但血管渗透性更好。在一起,我们的结果表明,OMNIA有助于精确和可靠地评估NP生物分布,以及将NP输送到肿瘤组织的机制研究。但是随着时间的流逝,来自脉管系统的NP的相对分布仍然非常稳定。比较45纳米和200纳米大小的NPs,相对于肝脏,较小的NPs在肿瘤中的积累较低,但血管渗透性更好。在一起,我们的结果表明,OMNIA有助于精确和可靠地评估NP生物分布,以及将NP递送至肿瘤组织的机制研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号