当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Synthesis of Chiral Allyl Carbamates via Merger of Photoredox and Nickel Catalysis
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-07 , DOI: 10.1002/adsc.202000404 Mateusz Garbacz 1 , Sebastian Stecko 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-07 , DOI: 10.1002/adsc.202000404 Mateusz Garbacz 1 , Sebastian Stecko 1
Affiliation
|
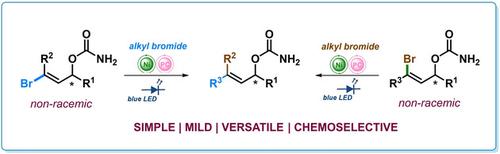
A mild, and versatile, organophotoredox/Ni‐mediated protocol was developed for the direct preparation of diverse, enantioenriched allyl carbamates. The reported approach represents a significant departure from classical step‐by‐step synthesis of allyl carbamates. This dual photoredox/Ni based strategy offers unrivalled capacity for convergent unification of readily available alkyl halides and chiral carbamates derived from 1‐bromo‐alken‐3‐ols with high chemoselectivity and efficiency. The reported photoredox/Ni catalyzed cross‐coupling reaction is not limited to carbamates, but also to other O‐derivatives such as esters, ethers, acetals, carbonates or silyl ethers. To demonstrate the utility of the reported protocol, the resulting allyl carbamates were transformed into functionalized non‐racemic allylamines through a sigmatropic rearrangement reaction in enantiospecific manner. This approach allowed for synthesis of enantiomeric allylamines by a simple control of the geometry of a double bond of allyl carbamates.
中文翻译:

光氧化还原和镍催化合成手性烯丙基氨基甲酸酯。
开发了一种温和且通用的有机光氧化还原/ Ni介导的方案,用于直接制备各种对映体富集的烯丙基氨基甲酸酯。报道的方法代表了烯丙基氨基甲酸酯经典一步一步合成的重大偏离。这种基于光氧化还原/镍的双重策略提供了无与伦比的能力,能够以较高的化学选择性和效率将衍生自1-溴代烯烃3-醇的烷基卤化物和手性氨基甲酸酯聚合统一。报道的光氧化还原/镍催化的交叉偶联反应不仅限于氨基甲酸酯,还包括其他O。衍生物,例如酯,醚,缩醛,碳酸酯或甲硅烷基醚。为了证明所报道方案的实用性,将所得的氨基甲酸烯丙酯通过对映体特异性方式的σ重排反应转化为功能化的非外消旋烯丙胺。该方法允许通过简单控制烯丙基氨基甲酸酯的双键的几何形状来合成对映异构烯丙基胺。
更新日期:2020-08-04
中文翻译:

光氧化还原和镍催化合成手性烯丙基氨基甲酸酯。
开发了一种温和且通用的有机光氧化还原/ Ni介导的方案,用于直接制备各种对映体富集的烯丙基氨基甲酸酯。报道的方法代表了烯丙基氨基甲酸酯经典一步一步合成的重大偏离。这种基于光氧化还原/镍的双重策略提供了无与伦比的能力,能够以较高的化学选择性和效率将衍生自1-溴代烯烃3-醇的烷基卤化物和手性氨基甲酸酯聚合统一。报道的光氧化还原/镍催化的交叉偶联反应不仅限于氨基甲酸酯,还包括其他O。衍生物,例如酯,醚,缩醛,碳酸酯或甲硅烷基醚。为了证明所报道方案的实用性,将所得的氨基甲酸烯丙酯通过对映体特异性方式的σ重排反应转化为功能化的非外消旋烯丙胺。该方法允许通过简单控制烯丙基氨基甲酸酯的双键的几何形状来合成对映异构烯丙基胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号