Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Close-up: HIV/SIV intasome structures shed new light on integrase inhibitor binding and viral escape mechanisms.
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-06-07 , DOI: 10.1111/febs.15438 Alan N Engelman 1, 2 , Peter Cherepanov 3, 4
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-06-07 , DOI: 10.1111/febs.15438 Alan N Engelman 1, 2 , Peter Cherepanov 3, 4
Affiliation
|
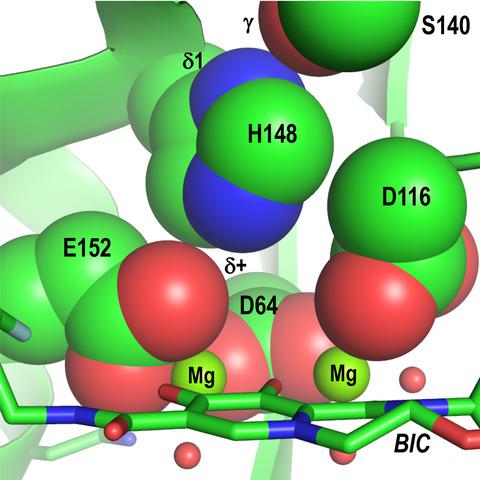
Integrase strand transfer inhibitors (INSTIs) are important components of drug formulations that are used to treat people living with HIV, and second‐generation INSTIs dolutegravir and bictegravir impart high barriers to the development of drug resistance. Reported 10 years ago, X‐ray crystal structures of prototype foamy virus (PFV) intasome complexes explained how INSTIs bind integrase to inhibit strand transfer activity and provided initial glimpses into mechanisms of drug resistance. However, comparatively low sequence identity between PFV and HIV‐1 integrases limited the depth of information that could be gleaned from the surrogate model system. Recent high‐resolution structures of HIV‐1 intasomes as well as intasomes from a closely related strain of simian immunodeficiency virus (SIV), which were determined using single‐particle cryogenic electron microscopy, have overcome this limitation. The new structures reveal the binding modes of several advanced INSTI compounds to the HIV/SIV integrase active site and critically inform the structural basis of drug resistance. These findings will help guide the continued development of this important class of antiretroviral therapeutics.
中文翻译:

特写:HIV/SIV 整合体结构揭示了整合酶抑制剂结合和病毒逃逸机制。
整合酶链转移抑制剂 (INSTI) 是用于治疗 HIV 感染者的药物制剂的重要组成部分,第二代 INSTI 多替拉韦和比替拉韦对耐药性的发展具有很高的障碍。10 年前报道,原型泡沫病毒 (PFV) 整合体复合物的 X 射线晶体结构解释了 INSTI 如何结合整合酶以抑制链转移活性,并提供了对耐药机制的初步了解。然而,PFV 和 HIV-1 整合酶之间相对较低的序列同一性限制了可以从替代模型系统中收集到的信息深度。HIV-1 整合体的最新高分辨率结构以及来自密切相关的猿猴免疫缺陷病毒 (SIV) 菌株的整合体,使用单粒子低温电子显微镜确定,克服了这一限制。新结构揭示了几种先进的 INSTI 化合物与 HIV/SIV 整合酶活性位点的结合模式,并为耐药性的结构基础提供了重要信息。这些发现将有助于指导这类重要的抗逆转录病毒疗法的持续发展。
更新日期:2020-06-07
中文翻译:

特写:HIV/SIV 整合体结构揭示了整合酶抑制剂结合和病毒逃逸机制。
整合酶链转移抑制剂 (INSTI) 是用于治疗 HIV 感染者的药物制剂的重要组成部分,第二代 INSTI 多替拉韦和比替拉韦对耐药性的发展具有很高的障碍。10 年前报道,原型泡沫病毒 (PFV) 整合体复合物的 X 射线晶体结构解释了 INSTI 如何结合整合酶以抑制链转移活性,并提供了对耐药机制的初步了解。然而,PFV 和 HIV-1 整合酶之间相对较低的序列同一性限制了可以从替代模型系统中收集到的信息深度。HIV-1 整合体的最新高分辨率结构以及来自密切相关的猿猴免疫缺陷病毒 (SIV) 菌株的整合体,使用单粒子低温电子显微镜确定,克服了这一限制。新结构揭示了几种先进的 INSTI 化合物与 HIV/SIV 整合酶活性位点的结合模式,并为耐药性的结构基础提供了重要信息。这些发现将有助于指导这类重要的抗逆转录病毒疗法的持续发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号