当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anionic behavior and single‐molecule crystal in fullerene confinements: A contribution from DFT energy decomposition and cooperativity analyses
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2020-06-07 , DOI: 10.1002/jcc.26362 Mojtaba Alipour 1 , Kimia Kargar 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2020-06-07 , DOI: 10.1002/jcc.26362 Mojtaba Alipour 1 , Kimia Kargar 1
Affiliation
|
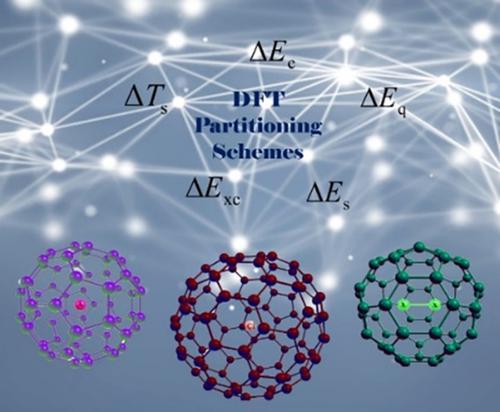
The recently proposed systems of various anions (A) confined inside C60, A− @ C60, which in turn behave as large and stable anions, (A @ C60)−, can find potential applications in various fields. On the other hand, it has earlier been shown that from the dihalogens (X2) encapsulated C60, X2 @ C60, only F2 @ C60 can be introduced as a system in which the cage acts as a cation C60+ and interacts with an endohedral anion, F2−, forming the F2− @ C60+ as a single‐molecule crystal compound. In this work, two density functional theory energy decomposition analysis (EDA) schemes, where in one of them the noninteracting kinetic, electrostatic, and exchange‐correlation energies come into play while another scheme, called as EDA‐SBL, includes the steric, electrostatic, and quantum effects as essential ingredients (S. Liu, J. Chem. Phys. 2007, 126, 244103), are utilized to find out what energetic components govern the unique characteristics of the (A @ C60)− and X2 @ C60 confinements. It is shown that the noninteracting kinetic energy and steric energies have important contributions to the total interaction energies for the considered systems. However, there are other confinements for which the electrostatic and exchange‐correlation contributions play also imperative roles. Furthermore, we find reasonable correlations between interaction energies and their components as well as the energetic components themselves, leading to an alternative EDA scheme including the noninteracting kinetic, steric, and electrostatic energies for investigations on other endohedral fullerenes. Extending our analyses to large size confinements, Cl− @ Cn with n up to 90 as illustrative examples, the quantitative cooperativity concept is also explored, where the positive and negative cooperativity profiles unveil a specific size of the anionic confinements to form the most stable large anion.
中文翻译:

富勒烯限制中的阴离子行为和单分子晶体:来自 DFT 能量分解和协同性分析的贡献
最近提出的各种阴离子 (A) 系统被限制在 C60 内,A-@C60,反过来又表现为大而稳定的阴离子,(A@C60)-,可以在各个领域找到潜在的应用。另一方面,早先已经表明,从二卤素 (X2) 封装的 C60、X2@C60 中,只有 F2@C60 可以作为系统引入,其中笼作为阳离子 C60+ 并与内嵌阴离子相互作用, F2-,形成 F2-@C60+ 作为单分子晶体化合物。在这项工作中,两个密度泛函理论能量分解分析 (EDA) 方案,其中一个方案是非相互作用的动能、静电和交换相关能发挥作用,而另一个方案,称为 EDA-SBL,包括空间、静电和量子效应作为基本成分(S. Liu, J. Chem. Phys. 2007, 126, 244103),被用来找出控制 (A @ C60)− 和 X2 @ C60 限制的独特特征的能量成分。结果表明,非相互作用动能和空间能对所考虑系统的总相互作用能有重要贡献。然而,还有其他限制,静电和交换相关贡献也起着重要作用。此外,我们发现相互作用能与其成分以及能量成分本身之间存在合理的相关性,从而产生了一种替代的 EDA 方案,包括非相互作用的动能、空间能和静电能,用于研究其他内嵌富勒烯。将我们的分析扩展到大尺寸限制,Cl−@Cn,n 高达 90 作为说明性示例,
更新日期:2020-06-07
中文翻译:

富勒烯限制中的阴离子行为和单分子晶体:来自 DFT 能量分解和协同性分析的贡献
最近提出的各种阴离子 (A) 系统被限制在 C60 内,A-@C60,反过来又表现为大而稳定的阴离子,(A@C60)-,可以在各个领域找到潜在的应用。另一方面,早先已经表明,从二卤素 (X2) 封装的 C60、X2@C60 中,只有 F2@C60 可以作为系统引入,其中笼作为阳离子 C60+ 并与内嵌阴离子相互作用, F2-,形成 F2-@C60+ 作为单分子晶体化合物。在这项工作中,两个密度泛函理论能量分解分析 (EDA) 方案,其中一个方案是非相互作用的动能、静电和交换相关能发挥作用,而另一个方案,称为 EDA-SBL,包括空间、静电和量子效应作为基本成分(S. Liu, J. Chem. Phys. 2007, 126, 244103),被用来找出控制 (A @ C60)− 和 X2 @ C60 限制的独特特征的能量成分。结果表明,非相互作用动能和空间能对所考虑系统的总相互作用能有重要贡献。然而,还有其他限制,静电和交换相关贡献也起着重要作用。此外,我们发现相互作用能与其成分以及能量成分本身之间存在合理的相关性,从而产生了一种替代的 EDA 方案,包括非相互作用的动能、空间能和静电能,用于研究其他内嵌富勒烯。将我们的分析扩展到大尺寸限制,Cl−@Cn,n 高达 90 作为说明性示例,











































 京公网安备 11010802027423号
京公网安备 11010802027423号