当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Is it possible to synthesize bulk salt compounds?
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-06-07 , DOI: 10.1002/qua.26276 Xiao‐Yong Yang 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-06-07 , DOI: 10.1002/qua.26276 Xiao‐Yong Yang 1
Affiliation
|
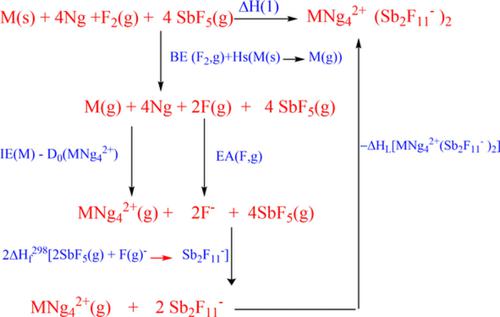
The existence and stability of  bulk salt compounds are theoretically investigated in this study. This undertaking is carried out to address the following challenge: synthesizing a bulk salt compound containing a noble gas lighter than Kr. The reliability of theoretical calculations on
bulk salt compounds are theoretically investigated in this study. This undertaking is carried out to address the following challenge: synthesizing a bulk salt compound containing a noble gas lighter than Kr. The reliability of theoretical calculations on  systems is assessed by benchmark calculations of the well‐known
systems is assessed by benchmark calculations of the well‐known  salt. In the benchmark calculations, a two‐pronged evaluation strategy, including direct and indirect evaluation methods, is used to theoretically investigate the spectroscopic constants of
salt. In the benchmark calculations, a two‐pronged evaluation strategy, including direct and indirect evaluation methods, is used to theoretically investigate the spectroscopic constants of  cation and the existence and stability of the
cation and the existence and stability of the  salt. The validity of the theoretical calculation methods in the benchmark calculations of
salt. The validity of the theoretical calculation methods in the benchmark calculations of  salt allows us to adopt a similar methodology to effectively predict the existence and stability of
salt allows us to adopt a similar methodology to effectively predict the existence and stability of  salt compounds. Calculations based on the Born‐Haber cycle using estimated lattice energies and some necessary ancillary thermochemical data show that
salt compounds. Calculations based on the Born‐Haber cycle using estimated lattice energies and some necessary ancillary thermochemical data show that  salt compounds can be synthesized, and their upper‐limit stable temperatures are estimated to be −237.589, −197.76, and −80.539°C. The
salt compounds can be synthesized, and their upper‐limit stable temperatures are estimated to be −237.589, −197.76, and −80.539°C. The  salt compound is the most promising candidate. Calculations also show that the
salt compound is the most promising candidate. Calculations also show that the  salt compounds cannot be stabilized.
salt compounds cannot be stabilized.
中文翻译:

有可能合成大量的盐化合物吗?
从 理论上研究了散装盐化合物的存在和稳定性。进行该任务以解决以下挑战:合成包含比Kr轻的稀有气体的本体盐化合物。
理论上研究了散装盐化合物的存在和稳定性。进行该任务以解决以下挑战:合成包含比Kr轻的稀有气体的本体盐化合物。 系统中理论计算的可靠性通过众所周知的
系统中理论计算的可靠性通过众所周知的 盐的基准计算进行评估。在基准计算中,从理论上研究了包括直接和间接评估方法在内的两管齐下的评估策略,以研究
盐的基准计算进行评估。在基准计算中,从理论上研究了包括直接和间接评估方法在内的两管齐下的评估策略,以研究 阳离子的光谱常数以及
阳离子的光谱常数以及 盐的存在和稳定性。理论计算方法在基准计算中的有效性。
盐的存在和稳定性。理论计算方法在基准计算中的有效性。 盐使我们可以采用类似的方法来有效地预测
盐使我们可以采用类似的方法来有效地预测 盐化合物的存在和稳定性。使用估算的晶格能量和一些必要的辅助热化学数据,基于Born-Haber循环进行的计算表明,
盐化合物的存在和稳定性。使用估算的晶格能量和一些必要的辅助热化学数据,基于Born-Haber循环进行的计算表明, 可以合成盐化合物,其稳定温度上限估计为-237.589,-197.76和-80.539°C。该
可以合成盐化合物,其稳定温度上限估计为-237.589,-197.76和-80.539°C。该 盐化合物是最有希望的候选人。计算还表明,
盐化合物是最有希望的候选人。计算还表明, 盐化合物无法稳定。
盐化合物无法稳定。
更新日期:2020-07-05
 bulk salt compounds are theoretically investigated in this study. This undertaking is carried out to address the following challenge: synthesizing a bulk salt compound containing a noble gas lighter than Kr. The reliability of theoretical calculations on
bulk salt compounds are theoretically investigated in this study. This undertaking is carried out to address the following challenge: synthesizing a bulk salt compound containing a noble gas lighter than Kr. The reliability of theoretical calculations on  systems is assessed by benchmark calculations of the well‐known
systems is assessed by benchmark calculations of the well‐known  salt. In the benchmark calculations, a two‐pronged evaluation strategy, including direct and indirect evaluation methods, is used to theoretically investigate the spectroscopic constants of
salt. In the benchmark calculations, a two‐pronged evaluation strategy, including direct and indirect evaluation methods, is used to theoretically investigate the spectroscopic constants of  cation and the existence and stability of the
cation and the existence and stability of the  salt. The validity of the theoretical calculation methods in the benchmark calculations of
salt. The validity of the theoretical calculation methods in the benchmark calculations of  salt allows us to adopt a similar methodology to effectively predict the existence and stability of
salt allows us to adopt a similar methodology to effectively predict the existence and stability of  salt compounds. Calculations based on the Born‐Haber cycle using estimated lattice energies and some necessary ancillary thermochemical data show that
salt compounds. Calculations based on the Born‐Haber cycle using estimated lattice energies and some necessary ancillary thermochemical data show that  salt compounds can be synthesized, and their upper‐limit stable temperatures are estimated to be −237.589, −197.76, and −80.539°C. The
salt compounds can be synthesized, and their upper‐limit stable temperatures are estimated to be −237.589, −197.76, and −80.539°C. The  salt compound is the most promising candidate. Calculations also show that the
salt compound is the most promising candidate. Calculations also show that the  salt compounds cannot be stabilized.
salt compounds cannot be stabilized.
中文翻译:

有可能合成大量的盐化合物吗?
从
 理论上研究了散装盐化合物的存在和稳定性。进行该任务以解决以下挑战:合成包含比Kr轻的稀有气体的本体盐化合物。
理论上研究了散装盐化合物的存在和稳定性。进行该任务以解决以下挑战:合成包含比Kr轻的稀有气体的本体盐化合物。 系统中理论计算的可靠性通过众所周知的
系统中理论计算的可靠性通过众所周知的 盐的基准计算进行评估。在基准计算中,从理论上研究了包括直接和间接评估方法在内的两管齐下的评估策略,以研究
盐的基准计算进行评估。在基准计算中,从理论上研究了包括直接和间接评估方法在内的两管齐下的评估策略,以研究 阳离子的光谱常数以及
阳离子的光谱常数以及 盐的存在和稳定性。理论计算方法在基准计算中的有效性。
盐的存在和稳定性。理论计算方法在基准计算中的有效性。 盐使我们可以采用类似的方法来有效地预测
盐使我们可以采用类似的方法来有效地预测 盐化合物的存在和稳定性。使用估算的晶格能量和一些必要的辅助热化学数据,基于Born-Haber循环进行的计算表明,
盐化合物的存在和稳定性。使用估算的晶格能量和一些必要的辅助热化学数据,基于Born-Haber循环进行的计算表明, 可以合成盐化合物,其稳定温度上限估计为-237.589,-197.76和-80.539°C。该
可以合成盐化合物,其稳定温度上限估计为-237.589,-197.76和-80.539°C。该 盐化合物是最有希望的候选人。计算还表明,
盐化合物是最有希望的候选人。计算还表明, 盐化合物无法稳定。
盐化合物无法稳定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号