当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular Nickel‐Catalyzed Ring‐Opening Reactions of Oxabenzonorbornadienes with C1‐Tethered Aryl Halides: An Improvement of Method
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-06-07 , DOI: 10.1002/ejoc.202000672 Samuel Koh 1 , Austin Pounder 1 , Elizabeth Brown 1 , William Tam 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-06-07 , DOI: 10.1002/ejoc.202000672 Samuel Koh 1 , Austin Pounder 1 , Elizabeth Brown 1 , William Tam 1
Affiliation
|
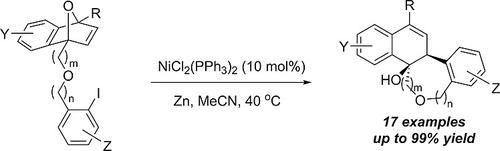
Oxabenzonorbornadienes with C1‐tethered aryl halides were found to rapidly undergo a ring‐opening reaction in the presence of NiCl2(PPh3)2, Zn, and MeCN to selectively form 1,2‐dihydronaphthalen‐1‐ol cores. 17 examples are shown with varying oxabenzonorbornadiene and iodoarene substitution, tether length, and halides, with yields up to 99 %.

中文翻译:

氧杂苯并降冰片二烯与C1系芳基卤化物的分子内镍催化开环反应:方法的改进
发现在NiCl 2(PPh 3)2,Zn和MeCN的存在下,带有C 1拴系的芳基卤化物的氧杂苯并降冰片二烯迅速发生开环反应,选择性地形成1,2-二氢萘-1-醇核。所示的17个实例具有不同的氧杂苯并降冰片二烯和碘芳烃取代基,系链长度和卤化物,产率高达99%。

更新日期:2020-08-03

中文翻译:

氧杂苯并降冰片二烯与C1系芳基卤化物的分子内镍催化开环反应:方法的改进
发现在NiCl 2(PPh 3)2,Zn和MeCN的存在下,带有C 1拴系的芳基卤化物的氧杂苯并降冰片二烯迅速发生开环反应,选择性地形成1,2-二氢萘-1-醇核。所示的17个实例具有不同的氧杂苯并降冰片二烯和碘芳烃取代基,系链长度和卤化物,产率高达99%。












































 京公网安备 11010802027423号
京公网安备 11010802027423号