当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Electrochemical α-Arylation of Cyclic β-Ketocarbonyls with Anodic Benzyne Intermediates.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-06-07 , DOI: 10.1002/anie.202006016 Longji Li 1, 2 , Yao Li 3 , Niankai Fu 1, 2 , Long Zhang 3 , Sanzhong Luo 1, 2, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-06-07 , DOI: 10.1002/anie.202006016 Longji Li 1, 2 , Yao Li 3 , Niankai Fu 1, 2 , Long Zhang 3 , Sanzhong Luo 1, 2, 3
Affiliation
|
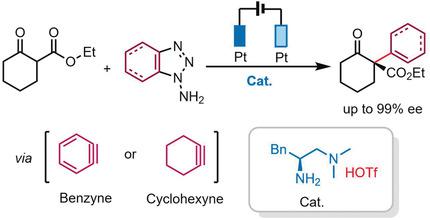
Asymmetric catalysis with benzyne remains elusive because of the highly fleeting and nonpolar nature of benzyne intermediates. Reported herein is an electrochemical approach for the oxidative generation of benzynes (cyclohexyne) and its successful merging with chiral primary aminocatalysis, formulating the first catalytic asymmetric enamine–benzyne (cyclohexyne) coupling reaction. Cobalt acetate was identified to stabilize the in situ generated arynes and facilitate its coupling with an enamine. This catalytic enamine‐benzyne protocol provides a concise method for the construction of diverse α‐aryl (α‐cyclohexenyl) quaternary carbon stereogenic centers with good stereoselectivities.
中文翻译:

β-酮羰基与阳极苄基中间体的催化不对称电化学α-芳基化反应。
由于苯炔中间体的高度瞬态和非极性性质,苯并炔的不对称催化作用仍然难以捉摸。本文报道的是一种电化学方法,用于氧化生成苯炔(环己炔)并将其成功与手性伯氨基催化反应合并,从而形成第一个催化不对称烯胺-苯并(环己炔)偶联反应。乙酸钴经鉴定可稳定原位生成的芳烃并促进其与烯胺的偶联。该催化的烯胺-苄基方案为构建具有良好立体选择性的各种α-芳基(α-环己烯基)季碳立构中心提供了一种简洁的方法。
更新日期:2020-08-10
中文翻译:

β-酮羰基与阳极苄基中间体的催化不对称电化学α-芳基化反应。
由于苯炔中间体的高度瞬态和非极性性质,苯并炔的不对称催化作用仍然难以捉摸。本文报道的是一种电化学方法,用于氧化生成苯炔(环己炔)并将其成功与手性伯氨基催化反应合并,从而形成第一个催化不对称烯胺-苯并(环己炔)偶联反应。乙酸钴经鉴定可稳定原位生成的芳烃并促进其与烯胺的偶联。该催化的烯胺-苄基方案为构建具有良好立体选择性的各种α-芳基(α-环己烯基)季碳立构中心提供了一种简洁的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号