当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Syntheses of (-)-Conidiogenone B, (-)-Conidiogenone, and (-)-Conidiogenol.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-06-05 , DOI: 10.1002/anie.202007247 Bo Xu 1 , Wen Xun 1 , Shaobin Su 1 , Hongbin Zhai 1, 2, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-06-05 , DOI: 10.1002/anie.202007247 Bo Xu 1 , Wen Xun 1 , Shaobin Su 1 , Hongbin Zhai 1, 2, 3
Affiliation
|
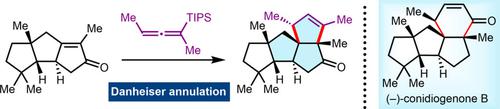
Cyclopianes are novel diterpenes featuring a highly strained 6/5/5/5 tetracyclic core embedded with 6–8 consecutive stereocenters. The concise total syntheses of (−)‐conidiogenone B, (−)‐conidiogenone, and (−)‐conidiogenol have been accomplished in 14–17 steps. The present work features a HAT‐mediated alkene–nitrile cyclization to access the cis‐biquinane, a Nicholas/Pauson–Khand reaction to construct the linear triquinane, and a Danheiser annulation to afford the congested angular triquinane skeleton.
中文翻译:

(-)-Conidiogenone B,(-)-Conidiogenone和(-)-Conidiogenol的总合成。
Cyclopianes是新颖的二萜,具有高度紧张的6/5/5/5四环核,嵌有6-8个连续的立体中心。(-)-conidiogenone B,(-)-conidiogenone和(-)-conidiogenol的简明全部合成过程已完成14-17个步骤。本工作的特点是通过HAT介导的烯烃-腈环化反应来获得顺式-二喹烷,通过Nicholas / Pauson-Khand反应来构建线性三喹烷,并通过Danheiser环合法获得了拥挤的角三喹烷骨架。
更新日期:2020-06-05
中文翻译:

(-)-Conidiogenone B,(-)-Conidiogenone和(-)-Conidiogenol的总合成。
Cyclopianes是新颖的二萜,具有高度紧张的6/5/5/5四环核,嵌有6-8个连续的立体中心。(-)-conidiogenone B,(-)-conidiogenone和(-)-conidiogenol的简明全部合成过程已完成14-17个步骤。本工作的特点是通过HAT介导的烯烃-腈环化反应来获得顺式-二喹烷,通过Nicholas / Pauson-Khand反应来构建线性三喹烷,并通过Danheiser环合法获得了拥挤的角三喹烷骨架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号