Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2020-06-06 , DOI: 10.1016/j.jfluchem.2020.109588 Ondřej Šimůnek , Markéta Rybáčková , Martin Svoboda , Jaroslav Kvíčala
|
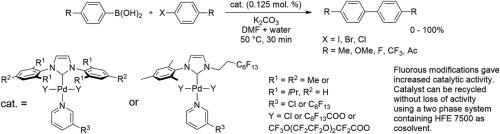
PEPPSI complexes are air and moisture stable Pd catalysts, which can be used conveniently in many coupling reactions. With the aim to obtain Pd catalysts recyclable by fluorous separation methods, we modified the structure of commercial PEPPSI complexes by per- or polyfluoroalkylation in various positions. The modifications included the use of a linear polyfluoroalkyl group instead of one aryl group on the NHC ligand, perfluoroalkylation of pyridine ligand, and substitution of chloride ligands on Pd for perfluoroalkanoates or perfluoropolyoxaalkanoates. Comparison of catalytic activity of commercial catalysts with the modified ones in Suzuki-Miyaura cross-coupling reactions showed that the fluorous modifications mostly resulted in the increase of catalytic activity. Moreover, polyfluoroalkylation enabled efficient medium fluorous recycle of the modified catalysts using a two phase aqueous DMF/HFE 7500 ether system.
中文翻译:

PEPPSI催化剂的氟类似物的合成,催化活性和中等氟再循环
PEPPSI配合物是空气和水分稳定的Pd催化剂,可方便地用于许多偶联反应中。为了获得可通过氟分离方法回收的Pd催化剂,我们通过在不同位置进行全氟烷基化或多氟烷基化改性了商用PEPPSI配合物的结构。修饰包括在NHC配体上使用线性多氟烷基代替一个芳基,吡啶配体的全氟烷基化,以及在Pd上用全氟链烷酸酯或全氟聚氧杂链烷酸酯取代氯配体。在铃木-宫浦的交叉偶联反应中,工业催化剂与改性催化剂的催化活性比较表明,氟的改性主要导致催化活性的增加。此外,



























 京公网安备 11010802027423号
京公网安备 11010802027423号