Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2020-06-07 , DOI: 10.1016/j.jaci.2020.05.032 Philippe Gevaert 1 , Theodore A Omachi 2 , Jonathan Corren 3 , Joaquim Mullol 4 , Joseph Han 5 , Stella E Lee 6 , Derrick Kaufman 2 , Monica Ligueros-Saylan 7 , Monet Howard 2 , Rui Zhu 2 , Ryan Owen 2 , Kit Wong 2 , Lutaf Islam 8 , Claus Bachert 9
|
Background
Chronic rhinosinusitis with nasal polyps (CRSwNP) is characterized by IgE hyperproduction and eosinophilic inflammation. The anti-IgE antibody, omalizumab, has demonstrated efficacy in patients with CRSwNP and comorbid asthma previously.
Objective
Our aim was to determine omalizumab safety and efficacy in CRSwNP in phase 3 trials (POLYP 1 and POLYP 2).
Methods
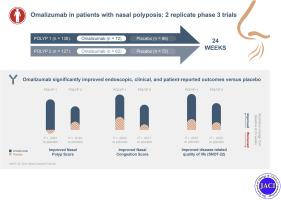
Adults with CRSwNP with inadequate response to intranasal corticosteroids were randomized (1:1) to omalizumab or placebo and intranasal mometasone for 24 weeks. Coprimary end points included change from baseline to week 24 in Nasal Polyp Score (NPS) and Nasal Congestion Score. Secondary end points included change from baseline to week 24 in Sino-Nasal Outcome Test-22 (SNOT-22) score, University of Pennsylvania Smell Identification Test, sense of smell, postnasal drip, runny nose, and adverse events.
Results
Patients in POLYP 1 (n = 138) and POLYP 2 (n = 127) exhibited severe CRSwNP and substantial quality of life impairment evidenced by a mean NPS higher than 6 and SNOT-22 score of approximately 60. Both studies met both the coprimary end points. SNOT-22 score, University of Pennsylvania Smell Identification Test score, sense of smell, postnasal drip, and runny nose were also significantly improved for omalizumab versus placebo. In POLYP 1 and POLYP 2, the mean changes from baseline at week 24 for omalizumab versus placebo were as follows: NPS, –1.08 versus 0.06 (P < .0001) and –0.90 versus –0.31 (P = .0140); Nasal Congestion Score, –0.89 versus –0.35 (P = .0004) and –0.70 versus –0.20 (P = .0017); and SNOT-22 score, –24.7 versus –8.6 (P < .0001) and –21.6 versus –6.6 (P < .0001). Adverse events were similar between groups.
Conclusion
Omalizumab significantly improved endoscopic, clinical, and patient-reported outcomes in severe CRSwNP with inadequate response to intranasal corticosteroids, and it was well tolerated.
中文翻译:

omalizumab在鼻息肉病中的疗效和安全性:2项随机3期试验。
背景
鼻息肉(CRSwNP)慢性鼻-鼻窦炎的特点是IgE高产和嗜酸性粒细胞炎症。抗IgE抗体omalizumab先前已在患有CRSwNP和合并症的患者中证明了疗效。
目的
我们的目标是在3期试验(POLYP 1和POLYP 2)中确定奥马珠单抗在CRSwNP中的安全性和有效性。
方法
将对鼻内皮质类固醇反应不足的CRSwNP成人分为omalizumab或安慰剂和鼻内莫米松随机(1:1),共24周。共主要终点包括从基线到第24周的鼻息肉评分(NPS)和鼻充血评分的变化。次要终点包括从鼻窦结果测试22(SNOT-22)评分从基线到第24周的变化,宾夕法尼亚大学气味识别测试,嗅觉,滴鼻后,流鼻涕和不良事件。
结果
POLYP 1(n = 138)和POLYP 2(n = 127)的患者表现出严重的CRSwNP和严重的生活质量下降,其平均NPS高于6,SNOT-22得分约为60。这两项研究均达到了共同主要终点点。与安慰剂相比,奥马珠单抗的SNOT-22得分,宾夕法尼亚大学气味识别测试得分,嗅觉,滴鼻液和流鼻涕也有明显改善。在POLYP 1和POLYP 2中,奥马珠单抗与安慰剂相比,从第24周开始的基线平均变化如下:NPS,–1.08 vs 0.06(P <.0001)和–0.90 vs –0.31(P = .0140);鼻充血评分,–0.89对–0.35(P = .0004)和–0.70对–0.20(P = .0017);和SNOT-22得分,分别为–24.7和–8.6(P <.0001)和–21.6与–6.6(P <.0001)。两组之间的不良事件相似。
结论
奥马珠单抗可显着改善重度CRSwNP患者的内镜,临床和患者报告的结局,且对鼻内皮质类固醇的反应不足,并且耐受性良好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号