Cell ( IF 45.5 ) Pub Date : 2020-06-06 , DOI: 10.1016/j.cell.2020.06.008 Hui Wang 1 , Yuntao Zhang 1 , Baoying Huang 2 , Wei Deng 3 , Yaru Quan 4 , Wenling Wang 2 , Wenbo Xu 2 , Yuxiu Zhao 1 , Na Li 1 , Jin Zhang 1 , Hongyang Liang 1 , Linlin Bao 3 , Yanfeng Xu 3 , Ling Ding 1 , Weimin Zhou 2 , Hong Gao 3 , Jiangning Liu 3 , Peihua Niu 2 , Li Zhao 2 , Wei Zhen 2 , Hui Fu 1 , Shouzhi Yu 1 , Zhengli Zhang 1 , Guangxue Xu 5 , Changgui Li 4 , Zhiyong Lou 5 , Miao Xu 4 , Chuan Qin 3 , Guizhen Wu 2 , George Fu Gao 2 , Wenjie Tan 2 , Xiaoming Yang 1
|
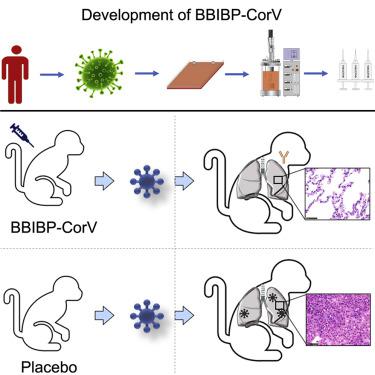
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) threatens global public health. The development of a vaccine is urgently needed for the prevention and control of COVID-19. Here, we report the pilot-scale production of an inactivated SARS-CoV-2 vaccine candidate (BBIBP-CorV) that induces high levels of neutralizing antibodies titers in mice, rats, guinea pigs, rabbits, and nonhuman primates (cynomolgus monkeys and rhesus macaques) to provide protection against SARS-CoV-2. Two-dose immunizations using 2 μg/dose of BBIBP-CorV provided highly efficient protection against SARS-CoV-2 intratracheal challenge in rhesus macaques, without detectable antibody-dependent enhancement of infection. In addition, BBIBP-CorV exhibits efficient productivity and good genetic stability for vaccine manufacture. These results support the further evaluation of BBIBP-CorV in a clinical trial.
中文翻译:

开发对 SARS-CoV-2 具有有效保护作用的候选灭活疫苗 BBIBP-CorV。
由严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 引起的 2019 年冠状病毒病 (COVID-19) 大流行威胁着全球公共卫生。预防和控制 COVID-19 迫切需要开发疫苗。在此,我们报告了一种灭活 SARS-CoV-2 候选疫苗 (BBIBP-CorV) 的中试规模生产,该候选疫苗可在小鼠、大鼠、豚鼠、兔子和非人类灵长类动物(食蟹猴和恒河猴)中诱导高水平的中和抗体滴度猕猴)以提供针对 SARS-CoV-2 的保护。使用 2 μg/剂量的 BBIBP-CorV 进行两剂免疫可为恒河猴提供针对 SARS-CoV-2 气管内攻击的高效保护,且未检测到抗体依赖性感染增强。此外,BBIBP-CorV 在疫苗生产方面表现出高效的生产力和良好的遗传稳定性。这些结果支持在临床试验中进一步评估 BBIBP-CorV。











































 京公网安备 11010802027423号
京公网安备 11010802027423号