当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed C8-H alkoxycarbonylation of 1-naphthylamines with alkyl chloroformates.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-05 , DOI: 10.1039/d0ob00586j Yaqi Shi 1 , Fan Yang 1 , Yangjie Wu 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-05 , DOI: 10.1039/d0ob00586j Yaqi Shi 1 , Fan Yang 1 , Yangjie Wu 1
Affiliation
|
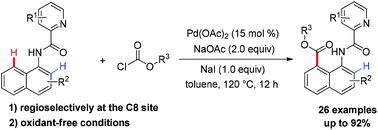
A simple and efficient protocol for palladium-catalyzed C8–H alkoxycarbonylation of 1-naphthylamine derivatives with alkyl chloroformates has been developed, exhibiting broad functional group tolerance, high regioselectivity, and oxidant-free conditions. Furthermore, the reaction features its ease of further functionalization and transformation. For example, the concise synthesis of one BET bromodomain inhibitor was accomplished via benz[cd]indol-2(1H)-one after multistep transformations from the obtained alkoxycarbonylation product. In addition, the control experiments suggest that the reaction might involve a radical process and the C–H bond cleavage might not be involved in the rate-determining step.
中文翻译:

1-萘胺与烷基氯甲酸酯的钯催化C8-H烷氧基羰基化反应。
已经开发了一种简单有效的方案,用于钯催化1-萘胺衍生物与烷基氯甲酸酯的C8–H烷氧基羰基化,具有宽泛的官能团耐受性,高区域选择性和无氧化剂条件。此外,该反应具有易于进一步官能化和转化的特征。例如,从获得的烷氧羰基化产物进行多步转化后,通过苯并[ cd ]吲哚-2(1 H)-1完成了一种BET溴结构域抑制剂的简明合成。另外,对照实验表明,该反应可能涉及自由基过程,而C–H键断裂可能不参与速率确定步骤。
更新日期:2020-06-24
中文翻译:

1-萘胺与烷基氯甲酸酯的钯催化C8-H烷氧基羰基化反应。
已经开发了一种简单有效的方案,用于钯催化1-萘胺衍生物与烷基氯甲酸酯的C8–H烷氧基羰基化,具有宽泛的官能团耐受性,高区域选择性和无氧化剂条件。此外,该反应具有易于进一步官能化和转化的特征。例如,从获得的烷氧羰基化产物进行多步转化后,通过苯并[ cd ]吲哚-2(1 H)-1完成了一种BET溴结构域抑制剂的简明合成。另外,对照实验表明,该反应可能涉及自由基过程,而C–H键断裂可能不参与速率确定步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号