当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning activation and self-immolative properties of the bioorthogonal alkene-azide click-and-release strategy.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-05 , DOI: 10.1039/d0ob00936a Jessica M Fairhall 1 , Madoka Murayasu 1 , Sumit Dadhwal 1 , Sarah Hook 1 , Allan B Gamble 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-05 , DOI: 10.1039/d0ob00936a Jessica M Fairhall 1 , Madoka Murayasu 1 , Sumit Dadhwal 1 , Sarah Hook 1 , Allan B Gamble 1
Affiliation
|
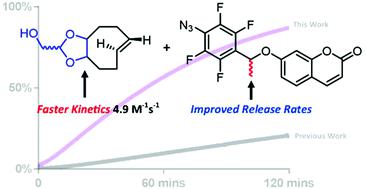
We report on a series of 4-azidobenzyloxy-substituted self-immolative linkers which undergo [3 + 2]-cycloaddition (click reaction) with functionalized trans-cyclooctenes (TCOs) at second-order rate constants in the range of 0.017 to 4.9 M−1 s−1. The choice of 4-azidobenzyloxy-substituted linker and the TCO play a critical role in the rate of all click-and-release steps, which includes the [3 + 2]-cycloaddition and subsequent degradation pathway of the triazoline to an aniline that undergoes 1,6- or 1,8-self-immolation of the phenol. We demonstrate that reacting a 4-azido-2,3,5,6-tetrafluorobenzyloxy-linker with a highly strained TCO (d-TCO) gives, to the best of our knowledge, the fastest TCO-strained alkene–azide click reaction to date (4.9 M−1 s−1), but with one caveat; release of phenol via 1,6-self-immolation is extremely slow. A methyl substituent attached to the benzyl carbon of this analogue maintains the rapid click-reaction rate, but has the added benefit of enabling the release of the phenol within hours. In an aqueous solvent at reagent concentrations in the micromolar range a maximium release was observed after 48 hours; ≈65 and ≈78% of phenol released depending on the TCO used. The new suite of linkers and their combination with TCOs of varying structure add to the toolbox of bioorthogonal click-and-release reactions.
中文翻译:

调整生物正交烯烃叠氮化物点击释放策略的活化和自焚特性。
我们报告了一系列的4-叠氮基苄氧基取代的自消灭性连接基,它们与官能化的反式环辛烯(TCO)进行[3 + 2]-环加成(点击反应),二级速率常数为0.017至4.9 M -1 s -1。4-叠氮基苄氧基取代的连接基和TCO的选择在所有单击和释放步骤的速率中都起着至关重要的作用,其中包括[3 + 2]-环加成以及随后的三唑啉向苯胺的降解途径苯酚的1,6-或1,8-自焚。我们证明,据我们所知,使4-叠氮基-2,3,5,6-四氟苄氧基连接基与高度拉紧的TCO(d-TCO)反应可产生最快的TCO拉长的烯烃-叠氮化物点击反应。日期(4.9 M -1s -1),但有一个警告;通过1,6-自焚的苯酚释放非常缓慢。与该类似物的苄基碳相连的甲基取代基可保持快速的点击反应速率,但具有在几小时内释放出苯酚的额外好处。在试剂浓度在微摩尔范围内的水性溶剂中,在48小时后观察到最大释放。取决于所使用的总拥有成本,≈65%和≈78%的苯酚释放出来。新的连接子套件以及它们与不同结构的TCO的结合增加了生物正交点击释放反应的工具箱。
更新日期:2020-07-01
中文翻译:

调整生物正交烯烃叠氮化物点击释放策略的活化和自焚特性。
我们报告了一系列的4-叠氮基苄氧基取代的自消灭性连接基,它们与官能化的反式环辛烯(TCO)进行[3 + 2]-环加成(点击反应),二级速率常数为0.017至4.9 M -1 s -1。4-叠氮基苄氧基取代的连接基和TCO的选择在所有单击和释放步骤的速率中都起着至关重要的作用,其中包括[3 + 2]-环加成以及随后的三唑啉向苯胺的降解途径苯酚的1,6-或1,8-自焚。我们证明,据我们所知,使4-叠氮基-2,3,5,6-四氟苄氧基连接基与高度拉紧的TCO(d-TCO)反应可产生最快的TCO拉长的烯烃-叠氮化物点击反应。日期(4.9 M -1s -1),但有一个警告;通过1,6-自焚的苯酚释放非常缓慢。与该类似物的苄基碳相连的甲基取代基可保持快速的点击反应速率,但具有在几小时内释放出苯酚的额外好处。在试剂浓度在微摩尔范围内的水性溶剂中,在48小时后观察到最大释放。取决于所使用的总拥有成本,≈65%和≈78%的苯酚释放出来。新的连接子套件以及它们与不同结构的TCO的结合增加了生物正交点击释放反应的工具箱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号