当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coagulating and flocculating ferrihydrite: application of zinc acetate salt
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-06-05 , DOI: 10.1039/d0ew00357c Samirul Islam 1, 2, 3, 4 , Sanjit Das 1, 2, 3, 4 , Geetanjali Mishra 1, 2, 3, 4 , Bidisa Das 2, 3, 4, 5, 6 , Arindam Malakar 1, 2, 3, 4 , Ilaria Carlomagno 7, 8, 9, 10 , Carlo Meneghini 7, 8, 9, 10 , Giovanni De Giudici 10, 11, 12, 13 , Liliana P. L. Gonçalves 14, 15, 16 , Juliana P. S. Sousa 14, 15, 16 , Yury V. Kolen'ko 14, 15, 16 , Andrei Cristian Kuncser 17, 18, 19, 20 , Sugata Ray 1, 2, 3, 4, 6
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-06-05 , DOI: 10.1039/d0ew00357c Samirul Islam 1, 2, 3, 4 , Sanjit Das 1, 2, 3, 4 , Geetanjali Mishra 1, 2, 3, 4 , Bidisa Das 2, 3, 4, 5, 6 , Arindam Malakar 1, 2, 3, 4 , Ilaria Carlomagno 7, 8, 9, 10 , Carlo Meneghini 7, 8, 9, 10 , Giovanni De Giudici 10, 11, 12, 13 , Liliana P. L. Gonçalves 14, 15, 16 , Juliana P. S. Sousa 14, 15, 16 , Yury V. Kolen'ko 14, 15, 16 , Andrei Cristian Kuncser 17, 18, 19, 20 , Sugata Ray 1, 2, 3, 4, 6
Affiliation
|
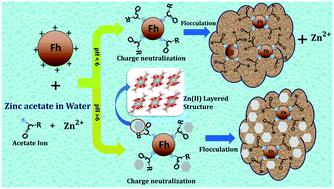
This paper outlines a method of extraction of iron from water in the form of iron oxyhydroxide natural nanoclusters at comparatively low concentrations and varied ranges of pH using zinc acetate salt. The zinc acetate salt dissociates into Zn2+ and acetate ions in water where Zn2+ interacts with iron clusters present in a solution of a given iron concentration and pH, while the acetate ion helps in charge-neutralization based coagulation and consequent precipitation of such nanoclusters. The Zn2+ ions may also lead to the growth of layered zinc hydroxide (LZH) nanosurfaces at pH ≥ 6 at sufficient loading. The advantage of this method is the active chemical interaction of Zn2+ with Fe clusters, followed by growth, which ensures that only some added Zn ions remain in the water while the rest precipitate out along with the residual iron oxyhydroxide, especially at higher pH. The solid that precipitated under various different conditions was successfully evaluated by XRD (formation of ferrihydrite-like nanoclusters (n-Fh)), FTIR (the presence of acetate in the solid n-Fh), TEM (the presence of zinc at higher pH), and EXAFS (local structural characterization). ICP analysis of the obtained solid and the corresponding filtrate revealed the removal efficiency of iron and zinc from the solution at various initial concentrations and pH values. This method of extracting soluble Fh-like nanoclusters by charge neutralization appears to be a suitable promising tool for water purification, because ferrihydrite is capable of isolating other adsorbed contaminants from water, along with itself.
中文翻译:

凝结和絮凝的水铁矿:醋酸锌盐的应用
本文概述了一种使用氢氧化锌盐以较低的浓度和不同的pH范围从羟基氧化铁天然纳米簇形式的水中提取铁的方法。乙酸锌盐在水中分解成Zn 2+和乙酸根离子,其中Zn 2+与存在于给定铁浓度和pH的溶液中的铁簇相互作用,而乙酸根离子则有助于基于电荷中和的凝聚作用和随后的沉淀纳米团簇。Zn 2+离子还可能在足够的负载下导致pH≥6的层状氢氧化锌(LZH)纳米表面的生长。该方法的优点是Zn 2+的活性化学相互作用Fe团簇随后生长,这确保了仅一些添加的Zn离子保留在水中,而其余的则与残留的羟基氧化铁一起沉淀出来,尤其是在较高的pH值下。通过XRD(亚铁酸盐样纳米团簇(n-Fh)的形成),FTIR(固体n-Fh中存在乙酸盐),TEM(在较高pH下存在锌)成功评估了在各种不同条件下沉淀的固体)和EXAFS(本地结构特征)。所得固体和相应滤液的ICP分析表明,在各种初始浓度和pH值下,铁和锌从溶液中的去除效率。这种通过电荷中和来提取可溶性Fh样纳米团簇的方法似乎是一种适合的有前途的水净化工具,
更新日期:2020-07-31
中文翻译:

凝结和絮凝的水铁矿:醋酸锌盐的应用
本文概述了一种使用氢氧化锌盐以较低的浓度和不同的pH范围从羟基氧化铁天然纳米簇形式的水中提取铁的方法。乙酸锌盐在水中分解成Zn 2+和乙酸根离子,其中Zn 2+与存在于给定铁浓度和pH的溶液中的铁簇相互作用,而乙酸根离子则有助于基于电荷中和的凝聚作用和随后的沉淀纳米团簇。Zn 2+离子还可能在足够的负载下导致pH≥6的层状氢氧化锌(LZH)纳米表面的生长。该方法的优点是Zn 2+的活性化学相互作用Fe团簇随后生长,这确保了仅一些添加的Zn离子保留在水中,而其余的则与残留的羟基氧化铁一起沉淀出来,尤其是在较高的pH值下。通过XRD(亚铁酸盐样纳米团簇(n-Fh)的形成),FTIR(固体n-Fh中存在乙酸盐),TEM(在较高pH下存在锌)成功评估了在各种不同条件下沉淀的固体)和EXAFS(本地结构特征)。所得固体和相应滤液的ICP分析表明,在各种初始浓度和pH值下,铁和锌从溶液中的去除效率。这种通过电荷中和来提取可溶性Fh样纳米团簇的方法似乎是一种适合的有前途的水净化工具,











































 京公网安备 11010802027423号
京公网安备 11010802027423号