当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coexistence of Left- and Right-Handed 12/10-Mixed Helices in Cyclically Constrained β-Peptides and Directed Formation of Single-Handed Helices upon Site-Specific Methylation.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.jpca.0c03545 Karl N Blodgett 1 , Geunhyuk Jang 2 , Sojung Kim 2 , Min Kyung Kim 2 , Soo Hyuk Choi 2 , Timothy S Zwier 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.jpca.0c03545 Karl N Blodgett 1 , Geunhyuk Jang 2 , Sojung Kim 2 , Min Kyung Kim 2 , Soo Hyuk Choi 2 , Timothy S Zwier 1
Affiliation
|
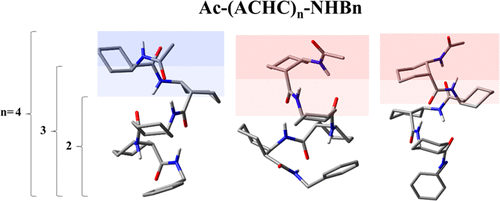
The inherent conformational preferences of the neutral β–peptide foldamer series, Ac-(ACHC)n-NHBn, n = 2–4, are studied in the gas phase using conformation-specific IR–UV double resonance methods. The cyclically constrained chiral β-amino acid cis-2-aminocyclohexane carboxylic acid (ACHC) is designed to bring both right- and left-handed helices into close energetic proximity. Comparison of the infrared spectra in the NH stretch and amide I/II regions with the predictions of DFT calculations lead to the unambiguous assignment of four out of the six observed conformations of the molecules in this series, while corroborating computational and spectral evidence, affords tentative assignments of the remaining two conformers for which IR data were not recorded. The observed structures fall into one of two conformational families: a right-handed 12/10-mixed helix or its “cap-disrupted” left-handed helical analogue, which coexist with significant populations. Site-specific and stereospecific methylation on the cyclohexane backbone at the dipeptide (n = 2) level is also tested as a means to sterically lock in a predetermined cyclohexane chair conformation. These substitutions are proven to be a means of selectively driving formation of one helical screw sense or the other. Calculated relative energies and free energies of all possible structures for the molecules provide strong supporting evidence that the rigid nature of the ACHC residue confers unusual stability to the 12/10-mixed helix conformation, regardless of local environment, temperature, or C-terminal capping unit. The simultaneous presence of both handed helices offers unique opportunities for future studies of their interconversion.
中文翻译:

循环约束的β肽中左手和右手12/10混合螺旋的共存和在特定位甲基化后单手螺旋的直接形成。
在气相中使用构象特异性的IR-UV双共振方法研究了中性β-肽折叠序列Ac-(ACHC)n -NHBn,n = 2-4的固有构象偏好。环状受限的手性β-氨基酸顺式-2-氨基环己烷羧酸(ACHC)旨在使右旋和左旋螺旋都处于紧密的能量附近。将NH延伸区和酰胺I / II区的红外光谱与DFT计算的预测结果进行比较,可以清楚地确定该系列六个分子中四个构象中的四个构象,同时证实了计算和光谱证据,具有初步意义未记录IR数据的其余两个构象子的分配。观察到的结构属于两个构象家族之一:右手12/10混合螺旋或与其“帽破坏”的左手螺旋类似物,它们与大量种群共存。二肽在环己烷主链上的位点特异性和立体特异性甲基化(n= 2)还测试了水平,将其空间锁定在预定的环己烷椅子构象中。事实证明,这些替代方法是选择性驱动形成一个螺旋螺牙或另一个螺牙的一种手段。分子所有可能结构的相对能和自由能的计算提供了有力的支持证据,表明ACHC残基的刚性性质赋予12/10混合螺旋构象异常的稳定性,而不受局部环境,温度或C端封端的影响单元。两个螺旋的同时存在为以后的相互转化研究提供了独特的机会。
更新日期:2020-07-16
中文翻译:

循环约束的β肽中左手和右手12/10混合螺旋的共存和在特定位甲基化后单手螺旋的直接形成。
在气相中使用构象特异性的IR-UV双共振方法研究了中性β-肽折叠序列Ac-(ACHC)n -NHBn,n = 2-4的固有构象偏好。环状受限的手性β-氨基酸顺式-2-氨基环己烷羧酸(ACHC)旨在使右旋和左旋螺旋都处于紧密的能量附近。将NH延伸区和酰胺I / II区的红外光谱与DFT计算的预测结果进行比较,可以清楚地确定该系列六个分子中四个构象中的四个构象,同时证实了计算和光谱证据,具有初步意义未记录IR数据的其余两个构象子的分配。观察到的结构属于两个构象家族之一:右手12/10混合螺旋或与其“帽破坏”的左手螺旋类似物,它们与大量种群共存。二肽在环己烷主链上的位点特异性和立体特异性甲基化(n= 2)还测试了水平,将其空间锁定在预定的环己烷椅子构象中。事实证明,这些替代方法是选择性驱动形成一个螺旋螺牙或另一个螺牙的一种手段。分子所有可能结构的相对能和自由能的计算提供了有力的支持证据,表明ACHC残基的刚性性质赋予12/10混合螺旋构象异常的稳定性,而不受局部环境,温度或C端封端的影响单元。两个螺旋的同时存在为以后的相互转化研究提供了独特的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号