当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium(II)-Catalyzed Annulative Coupling of β-Ketothioamides with α-Diazo Compounds: Access to Highly Functionalized Thiazolidin-4-ones and Thiazolines.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.joc.0c00378 Monish Arbaz Ansari 1 , Dhananjay Yadav 1 , Maya Shankar Singh 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.joc.0c00378 Monish Arbaz Ansari 1 , Dhananjay Yadav 1 , Maya Shankar Singh 1
Affiliation
|
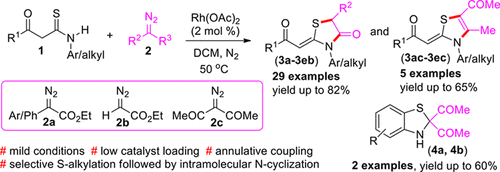
An operationally simple and efficient one-pot protocol for the synthesis of highly functionalized thiazolidin-4-ones and thiazolines has been devised via Rh(OAc)2-catalyzed annulative coupling of β-ketothioamides with diazo compounds under mild reaction conditions for the first time. This double functionalization of diazo compounds proceeds via selective S-alkylation followed by intramolecular N-cyclization enabling the formation of C–S and C–N bonds at moderate temperature. Notably, the products possess Z-stereochemistry with regard to the exocyclic C═C double bond at the 2-position of the ring. Further, the synthetic utility of the strategy has been revealed to access 2,3-dihydrobenzo[d]thiazoles. Remarkably, atom economy and tolerance of a wide range of functional groups are added characteristics to this strategy.
中文翻译:

铑(II)催化的β-酮硫酰胺与α-重氮化合物的环形偶联:获得高度官能化的噻唑烷丁-4-酮和噻唑啉。
首次在温和的反应条件下,通过Rh(OAc)2催化β-酮硫酰胺与重氮化合物的环状偶联,设计了一种操作简单且有效的一锅操作方案,用于合成高度功能化的噻唑烷酮-4-酮和噻唑啉。重氮化合物的这种双重功能化是通过选择性的S-烷基化,然后进行分子内的N-环化来实现的,从而在中等温度下形成C–S和C–N键。值得注意的是,产物在环的2-位上的环外C═C双键具有Z-立体化学。此外,该策略的综合效用已显示出可用于2,3-二氢苯并[ d]。噻唑类。值得注意的是,原子经济性和对各种官能团的耐受性是该策略的附加特征。
更新日期:2020-07-02
中文翻译:

铑(II)催化的β-酮硫酰胺与α-重氮化合物的环形偶联:获得高度官能化的噻唑烷丁-4-酮和噻唑啉。
首次在温和的反应条件下,通过Rh(OAc)2催化β-酮硫酰胺与重氮化合物的环状偶联,设计了一种操作简单且有效的一锅操作方案,用于合成高度功能化的噻唑烷酮-4-酮和噻唑啉。重氮化合物的这种双重功能化是通过选择性的S-烷基化,然后进行分子内的N-环化来实现的,从而在中等温度下形成C–S和C–N键。值得注意的是,产物在环的2-位上的环外C═C双键具有Z-立体化学。此外,该策略的综合效用已显示出可用于2,3-二氢苯并[ d]。噻唑类。值得注意的是,原子经济性和对各种官能团的耐受性是该策略的附加特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号