当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral NCN Pincer Iridium(III) Complexes with Bis(imidazolinyl)phenyl Ligands: Synthesis and Application in Enantioselective C–H Functionalization of Indoles with α-Aryl-α-diazoacetates
Organometallics ( IF 2.5 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.organomet.0c00174 Nan Li 1 , Wen-Jing Zhu 1 , Juan-Juan Huang 1 , Xin-Qi Hao 1 , Jun-Fang Gong 1 , Mao-Ping Song 1
Organometallics ( IF 2.5 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.organomet.0c00174 Nan Li 1 , Wen-Jing Zhu 1 , Juan-Juan Huang 1 , Xin-Qi Hao 1 , Jun-Fang Gong 1 , Mao-Ping Song 1
Affiliation
|
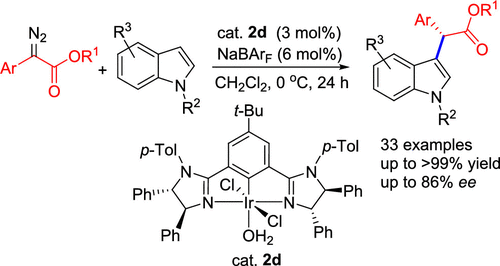
A series of chiral NCN pincer iridium(III) complexes 2a–h with bis(imidazolinyl)phenyl ligands were synthesized via the central aryl C2–H bond activation of the 1,3-bis(2′-imidazolinyl)benzene ligands. The incorporation of a 5-tert-butyl group into the central aryl ring of the ligands was found to markedly improve the efficiency of the desired C2-metalation, leading to an obvious enhancement in the yields of the Ir(III) complexes. Consequently, complexes with a tert-butyl group on the central aryl ring were obtained in 34–47% yields, whereas those without the group were produced in only 13–16% yields. All of the new complexes have been characterized by elemental analysis and 1H and 13C{1H} NMR spectroscopy. In addition, the molecular structures of complexes 2c, 2d, and 2g′ have been determined by X-ray single-crystal diffraction. 2c and 2d are, indeed, the anticipated six-coordinate pincer Ir(III) complexes. In contrast, 2g′ is a coordinatively unsaturated five-coordinate pincer Ir(III) complex. The Ir(III) complexes were used as the catalysts for the asymmetric C–H insertion reaction of α-aryl-α-diazoacetates with N-protected indoles. With a catalyst loading of 3 mol % and in the presence of 6 mol % of NaBArF, a variety of optically active indole derivatives bearing chiral functional groups at the C3 position were obtained in good yields with moderate to good enantioselectivities (up to 86% ee).
中文翻译:

双(咪唑啉基)苯基配体的手性NCN钳铱(III)配合物:合成和在吲哚α-芳基-α-重氮乙酸酯对映体CH官能化中的应用
通过对1,3-双(2'-咪唑啉基)苯配体的中心芳基C2-H键活化,合成了具有双(咪唑啉基)苯基配体的一系列手性NCN钳形铱(III)配合物2a – h。发现将5-叔丁基结合到配体的中心芳基环中可显着提高所需的C 2-金属化的效率,从而导致Ir(III)配合物的产率明显提高。因此,在中心芳基环上具有叔丁基的配合物的收率为34–47%,而没有该基团的配合物的收率仅为13–16%。所有新的配合物均已通过元素分析和1 H和13表征C { 1 H} NMR光谱。另外,已经通过X射线单晶衍射确定了络合物2c,2d和2g'的分子结构。2c和2d实际上是预期的六坐标钳式Ir(III)配合物。相反,2g'是配位不饱和的五坐标钳子Ir(III)络合物。Ir(III)络合物用作α-芳基-α-重氮乙酸酯与N保护的吲哚的不对称CH插入反应的催化剂。在3 mol%的催化剂负载量和6 mol%的NaBAr F存在下,以良好的收率获得了具有中等至良好对映选择性(高达86%ee)的各种旋光活性吲哚衍生物,它们在C3位带有手性官能团。
更新日期:2020-06-23
中文翻译:

双(咪唑啉基)苯基配体的手性NCN钳铱(III)配合物:合成和在吲哚α-芳基-α-重氮乙酸酯对映体CH官能化中的应用
通过对1,3-双(2'-咪唑啉基)苯配体的中心芳基C2-H键活化,合成了具有双(咪唑啉基)苯基配体的一系列手性NCN钳形铱(III)配合物2a – h。发现将5-叔丁基结合到配体的中心芳基环中可显着提高所需的C 2-金属化的效率,从而导致Ir(III)配合物的产率明显提高。因此,在中心芳基环上具有叔丁基的配合物的收率为34–47%,而没有该基团的配合物的收率仅为13–16%。所有新的配合物均已通过元素分析和1 H和13表征C { 1 H} NMR光谱。另外,已经通过X射线单晶衍射确定了络合物2c,2d和2g'的分子结构。2c和2d实际上是预期的六坐标钳式Ir(III)配合物。相反,2g'是配位不饱和的五坐标钳子Ir(III)络合物。Ir(III)络合物用作α-芳基-α-重氮乙酸酯与N保护的吲哚的不对称CH插入反应的催化剂。在3 mol%的催化剂负载量和6 mol%的NaBAr F存在下,以良好的收率获得了具有中等至良好对映选择性(高达86%ee)的各种旋光活性吲哚衍生物,它们在C3位带有手性官能团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号