当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the Promising Potential of High Permeation Vesicle-Mediated Localized Transdermal Delivery of Docetaxel in Breast Cancer To Overcome the Limitations of Systemic Chemotherapy.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.molpharmaceut.0c00211 Minal Bathara 1 , Tushar Date 1 , Dasharath Chaudhari 1 , Rohan Ghadi 1 , Kaushik Kuche 1 , Sanyog Jain 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.molpharmaceut.0c00211 Minal Bathara 1 , Tushar Date 1 , Dasharath Chaudhari 1 , Rohan Ghadi 1 , Kaushik Kuche 1 , Sanyog Jain 1
Affiliation
|
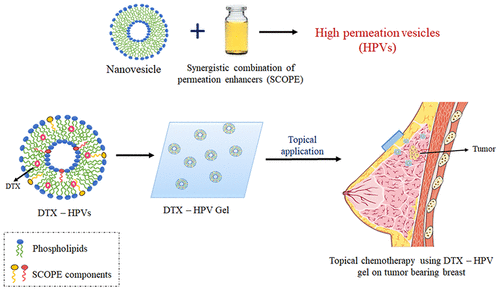
The currently available systemic chemotherapy for treating breast cancer often results in serious systemic side effects and compromises patient compliance. The distinct anatomical features of human breasts (e.g., embryological origin of breast skin, highly developed internal lymphatic and venous circulation, and the presence of mammary fat layers) help in preferential accumulation of drugs into breasts after topical application on breast region. This unique feature is termed as localized transdermal delivery which could be utilized for effectively delivering anticancer agents to treat breast cancer and reducing the systemic side effects by limiting their presence in blood. However, the clinical effectiveness of this drug delivery approach is highly limited by barrier properties of skin reducing the permeation of anticancer drugs. In the present work, we have developed high permeation vesicles (HPVs) using phospholipids and synergistic combination of permeation enhancers (SCOPE) to improve the skin permeation of drugs. Docetaxel (DTX) was used as a model drug for hypothesis testing. The optimized SCOPE mixture composed of sodium oleate/sodium lauryl ether sulfate/propylene glycol in 64:16:20% w/w ratio. DTX HPVs were prepared using phospholipid: SCOPE, 8:2% w/w ratio. DTX HPVs exhibited as a uniform deformable vesicles with size range 124.2 ± 7.6 nm, significantly improved skin permeation profile, and sustained drug release until 48 h. Superior vesicle deformability, better vesicle membrane fluidization, and SCOPE mediated enhancement in skin fluidization were the prime factors behind enhancement of DTX permeation. The improved cellular uptake, reduced IC50 values, and higher apoptotic index of DTX HPVs in MCF-7 and MDA-MB-231 cells ensured the therapeutic effectiveness of HPV based therapy. Also, HPVs were found to be predominantly internalized inside cells through clathrin and caveolae-dependent endocytic pathways. Bioimaging analysis in mice confirmed the tumor penetration potential and effective accumulation of HPVs inside tumors after topical application. In vivo studies were carried out in comparison with marketed intravenous DTX injection (Taxotere) to compare the effectiveness of topical chemotherapy. The topical application of DTX HPV gel in tumor bearing mice resulted in nearly 4-fold tumor volume reduction which was equivalent to intravenous Taxotere therapy. Toxicity analysis of DTX HPV gel in comparison with intravenous Taxotere dosing showcased remarkably lower levels of toxicity biomarkers (aspartate transaminase (AST), alanine transaminase (ALT), blood urea nitrogen (BUN), and creatinine), indicating improved safety of topical chemotherapy. Overall results warranted the effectiveness of topical DTX chemotherapy to reduce tumor burden with substantially reduced risk of systemic toxicities in breast cancer.
中文翻译:

探索多西紫杉醇在乳腺癌中高渗透性囊泡介导的局部透皮递送的潜在潜力,以克服全身化疗的局限性。
当前可利用的用于治疗乳腺癌的全身化学疗法经常导致严重的全身副作用并损害患者的依从性。人乳房的独特解剖特征(例如,乳房皮肤的胚胎学起源,高度发达的内部淋巴和静脉循环以及乳腺脂肪层的存在)有助于在乳房区域局部应用后优先向乳房内积聚药物。该独特特征被称为局部透皮递送,其可用于有效递送抗癌剂以治疗乳腺癌并通过限制它们在血液中的存在来减少全身性副作用。然而,这种药物递送方法的临床有效性在很大程度上受到皮肤屏障性能的限制,从而降低了抗癌药物的渗透性。在目前的工作中,我们已经开发出使用磷脂和渗透促进剂(SCOPE)的协同组合来改善药物的皮肤渗透的高渗透囊泡(HPV)。多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 我们使用磷脂和渗透促进剂(SCOPE)的协同组合开发了高渗透性囊泡(HPV),以改善药物的皮肤渗透性。多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 我们使用磷脂和渗透促进剂(SCOPE)的协同组合开发了高渗透性囊泡(HPV),以改善药物的皮肤渗透性。多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC50DTX HPV在MCF-7和MDA-MB-231细胞中的较高的细胞凋亡值和较高的凋亡指数确保了基于HPV的治疗的疗效。同样,发现HPV主要通过网格蛋白和小窝依赖的胞吞途径内化在细胞内。小鼠的生物成像分析证实了局部应用后,肿瘤具有潜在的穿透力和HPV的有效积累。与市售静脉注射DTX注射液(Taxotere)进行了体内研究,以比较局部化疗的有效性。DTX HPV凝胶在荷瘤小鼠中的局部应用导致肿瘤体积缩小近4倍,这相当于静脉使用Taxotere治疗。DTX HPV凝胶的毒性分析与静脉给予Taxotere剂量相比,毒性生物标志物(天冬氨酸转氨酶(AST),丙氨酸转氨酶(ALT),血尿素氮(BUN)和肌酐)水平显着降低,表明局部化疗的安全性得到了改善。总体结果证明,局部DTX化疗可有效降低肿瘤负担,并显着降低乳腺癌的全身毒性风险。
更新日期:2020-07-06
中文翻译:

探索多西紫杉醇在乳腺癌中高渗透性囊泡介导的局部透皮递送的潜在潜力,以克服全身化疗的局限性。
当前可利用的用于治疗乳腺癌的全身化学疗法经常导致严重的全身副作用并损害患者的依从性。人乳房的独特解剖特征(例如,乳房皮肤的胚胎学起源,高度发达的内部淋巴和静脉循环以及乳腺脂肪层的存在)有助于在乳房区域局部应用后优先向乳房内积聚药物。该独特特征被称为局部透皮递送,其可用于有效递送抗癌剂以治疗乳腺癌并通过限制它们在血液中的存在来减少全身性副作用。然而,这种药物递送方法的临床有效性在很大程度上受到皮肤屏障性能的限制,从而降低了抗癌药物的渗透性。在目前的工作中,我们已经开发出使用磷脂和渗透促进剂(SCOPE)的协同组合来改善药物的皮肤渗透的高渗透囊泡(HPV)。多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 我们使用磷脂和渗透促进剂(SCOPE)的协同组合开发了高渗透性囊泡(HPV),以改善药物的皮肤渗透性。多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 我们使用磷脂和渗透促进剂(SCOPE)的协同组合开发了高渗透性囊泡(HPV),以改善药物的皮肤渗透性。多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 多西他赛(DTX)被用作模型药物进行假设检验。优化的SCOPE混合物由油酸钠/十二烷基醚硫酸钠/丙二醇组成,比例为64:16:20%w / w。使用磷脂:SCOPE,8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC 使用磷脂:SCOPE,以8:2%w / w的比例制备DTX HPV。DTX HPV表现为大小范围为124.2±7.6 nm的均匀可变形囊泡,可显着改善皮肤渗透性,并持续释放药物直至48 h。优异的囊泡可变形性,更好的囊泡膜流化以及SCOPE介导的皮肤流化增强是DTX渗透增强的主要因素。改善细胞吸收,减少IC50DTX HPV在MCF-7和MDA-MB-231细胞中的较高的细胞凋亡值和较高的凋亡指数确保了基于HPV的治疗的疗效。同样,发现HPV主要通过网格蛋白和小窝依赖的胞吞途径内化在细胞内。小鼠的生物成像分析证实了局部应用后,肿瘤具有潜在的穿透力和HPV的有效积累。与市售静脉注射DTX注射液(Taxotere)进行了体内研究,以比较局部化疗的有效性。DTX HPV凝胶在荷瘤小鼠中的局部应用导致肿瘤体积缩小近4倍,这相当于静脉使用Taxotere治疗。DTX HPV凝胶的毒性分析与静脉给予Taxotere剂量相比,毒性生物标志物(天冬氨酸转氨酶(AST),丙氨酸转氨酶(ALT),血尿素氮(BUN)和肌酐)水平显着降低,表明局部化疗的安全性得到了改善。总体结果证明,局部DTX化疗可有效降低肿瘤负担,并显着降低乳腺癌的全身毒性风险。



























 京公网安备 11010802027423号
京公网安备 11010802027423号