Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding Mono- and Bivalent Ion Selectivities of Nanoporous Graphene Using Ionic and Bi-ionic Potentials.
Langmuir ( IF 3.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.langmuir.0c00924 Mandakranta Ghosh 1 , Lukas Madauß 2 , Marika Schleberger 2 , Henning Lebius 3 , Abdenacer Benyagoub 3 , Jeffery A Wood 1 , Rob G H Lammertink 1
Langmuir ( IF 3.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.langmuir.0c00924 Mandakranta Ghosh 1 , Lukas Madauß 2 , Marika Schleberger 2 , Henning Lebius 3 , Abdenacer Benyagoub 3 , Jeffery A Wood 1 , Rob G H Lammertink 1
Affiliation
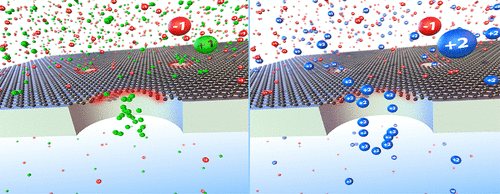
|
Nanoporous graphene displays salt-dependent ion permeation. In this work, we investigate the differences in Donnan potentials arising between reservoirs, separated by a perforated graphene membrane, containing different cations. We compare the case of monovalent cations interacting with nanoporous graphene with the case of bivalent cations. This is accomplished through both measurements of membrane potential arising between two salt reservoirs at different concentrations involving a single cation (ionic potential) and between two reservoirs containing different cations at the same concentration (bi-ionic potential). In our present study, Donnan dialysis experiments involve bivalent MgCl2, CaCl2, and CuCl2 as well as monovalent KCl and NH4Cl salts. For all salts, except CuCl2, clear Donnan and diffusion potential plateaus were observed at low and high salt concentrations, respectively. Our observations show that the membrane potential scaled to the Nernst potential for bivalent cations has a lower value (≈50%) than for monovalent cations (≈72%) in the Donnan exclusion regime. This is likely due to the adsorption of these bivalent cations on monolayer graphene. For bivalent cations, the diffusion regime is reached at a lower ionic strength compared to the monovalent cations. For Mg2+ and Ca2+, the membrane potential does not seem to depend upon the type of ions in the entire ionic strength range. A similar behavior is observed for the KCl and NH4Cl membrane potential curves. For CuCl2, the membrane potential curve is shifted toward lower ionic strength compared to the other two bivalent salts and the Donnan plateau is not observed at the lowest ionic strength. Bi-ionic potential measurements give further insight into the strength of specific interactions, allowing for the estimation of the relative ionic selectivities of different cations based on comparing their bi-ionic potentials. This effect of possible ion adsorption on graphene can be removed through ion exchange with monovalent salts.
中文翻译:

使用离子和双离子电势了解纳米多孔石墨烯的单价和二价离子选择性。
纳米多孔石墨烯显示出盐依赖性离子渗透。在这项工作中,我们调查了由含不同阳离子的多孔石墨烯膜隔开的储层之间产生的Donnan电位的差异。我们比较了单价阳离子与纳米多孔石墨烯相互作用的情况和二价阳离子的情况。这是通过测量两个盐储集层之间在不同浓度下涉及单个阳离子的膜电位(离子电势)和两个包含相同浓度不同阳离子的储罐之间的膜电位(双离子电势)来完成的。在本研究中,Donnan透析实验涉及二价MgCl 2,CaCl 2和CuCl 2以及一价KCl和NH 4Cl盐。对于所有盐,除了CuCl 2以外,在低和高盐浓度下均观察到明显的Donnan和扩散势平台。我们的观察结果表明,在Donnan排斥体系中,与二价阳离子相比,按Nernst电位换算的膜电位具有较低的值(≈50%)。这可能是由于这些二价阳离子在单层石墨烯上的吸附。对于二价阳离子,与一价阳离子相比,在较低的离子强度下达到了扩散状态。对于Mg 2+和Ca 2+,膜电位似乎并不取决于整个离子强度范围内的离子类型。对于KCl和NH 4观察到类似的行为Cl膜电位曲线。对于CuCl 2,与其他两种二价盐相比,膜电势曲线朝着较低的离子强度方向移动,并且在最低的离子强度下未观察到Donnan平稳期。双向离子电势测量可进一步洞察特定相互作用的强度,从而可以根据比较它们的双向离子电势来估算不同阳离子的相对离子选择性。可以通过与单价盐进行离子交换来消除可能的离子吸附在石墨烯上的这种影响。
更新日期:2020-07-07
中文翻译:

使用离子和双离子电势了解纳米多孔石墨烯的单价和二价离子选择性。
纳米多孔石墨烯显示出盐依赖性离子渗透。在这项工作中,我们调查了由含不同阳离子的多孔石墨烯膜隔开的储层之间产生的Donnan电位的差异。我们比较了单价阳离子与纳米多孔石墨烯相互作用的情况和二价阳离子的情况。这是通过测量两个盐储集层之间在不同浓度下涉及单个阳离子的膜电位(离子电势)和两个包含相同浓度不同阳离子的储罐之间的膜电位(双离子电势)来完成的。在本研究中,Donnan透析实验涉及二价MgCl 2,CaCl 2和CuCl 2以及一价KCl和NH 4Cl盐。对于所有盐,除了CuCl 2以外,在低和高盐浓度下均观察到明显的Donnan和扩散势平台。我们的观察结果表明,在Donnan排斥体系中,与二价阳离子相比,按Nernst电位换算的膜电位具有较低的值(≈50%)。这可能是由于这些二价阳离子在单层石墨烯上的吸附。对于二价阳离子,与一价阳离子相比,在较低的离子强度下达到了扩散状态。对于Mg 2+和Ca 2+,膜电位似乎并不取决于整个离子强度范围内的离子类型。对于KCl和NH 4观察到类似的行为Cl膜电位曲线。对于CuCl 2,与其他两种二价盐相比,膜电势曲线朝着较低的离子强度方向移动,并且在最低的离子强度下未观察到Donnan平稳期。双向离子电势测量可进一步洞察特定相互作用的强度,从而可以根据比较它们的双向离子电势来估算不同阳离子的相对离子选择性。可以通过与单价盐进行离子交换来消除可能的离子吸附在石墨烯上的这种影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号