当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proximal Biotinylation-Based Combinatory Approach for Isolating Integral Plasma Membrane Proteins.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.jproteome.0c00113 Mehmet Akdag 1 , Zeynep Sabahat Yunt 1 , Altug Kamacioglu 1 , Mohammed Haroon Qureshi 1 , Busra A Akarlar 1 , Nurhan Ozlu 1
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.jproteome.0c00113 Mehmet Akdag 1 , Zeynep Sabahat Yunt 1 , Altug Kamacioglu 1 , Mohammed Haroon Qureshi 1 , Busra A Akarlar 1 , Nurhan Ozlu 1
Affiliation
|
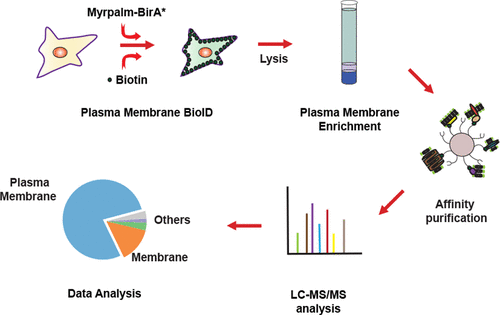
Comprehensive profiling of the cell-surface proteome has been challenging due to the lack of tools for an effective and reproducible way to isolate plasma membrane proteins from mammalian cells. Here we employ a proximity-dependent biotinylation approach to label and isolate plasma membrane proteins without an extra in vitro labeling step, which we call Plasma Membrane-BioID. The lipid-modified BirA* enzyme (MyrPalm BirA*) was targeted to the inner leaflet of the plasma membrane, where it effectively biotinylated plasma membrane proteins. Biotinylated proteins were then affinity-purified and analyzed by mass spectrometry. Our analysis demonstrates that combining conventional sucrose density gradient centrifugation and Plasma Membrane-BioID is ideal to overcome the inherent limitations of the identification of integral membrane proteins, and it yields highly pure plasma components for downstream proteomic analysis.
中文翻译:

基于近端生物素化的组合方法分离整体血浆膜蛋白。
由于缺乏用于从哺乳动物细胞中分离质膜蛋白的有效且可再现的方法的工具,因此对细胞表面蛋白质组进行全面的分析一直具有挑战性。在这里,我们采用邻近依赖性生物素化方法来标记和分离质膜蛋白,而无需进行额外的体外操作标记步骤,我们称为血浆膜BioID。脂质修饰的BirA *酶(MyrPalm BirA *)靶向质膜的内部小叶,在那里它可以有效地生物素化质膜蛋白。然后将生物素化的蛋白质亲和纯化,并通过质谱分析。我们的分析表明,将常规的蔗糖密度梯度离心法与血浆膜BioID结合使用是克服鉴定完整膜蛋白的固有局限性的理想选择,并且可以产生用于下游蛋白质组学分析的高纯度血浆成分。
更新日期:2020-08-08
中文翻译:

基于近端生物素化的组合方法分离整体血浆膜蛋白。
由于缺乏用于从哺乳动物细胞中分离质膜蛋白的有效且可再现的方法的工具,因此对细胞表面蛋白质组进行全面的分析一直具有挑战性。在这里,我们采用邻近依赖性生物素化方法来标记和分离质膜蛋白,而无需进行额外的体外操作标记步骤,我们称为血浆膜BioID。脂质修饰的BirA *酶(MyrPalm BirA *)靶向质膜的内部小叶,在那里它可以有效地生物素化质膜蛋白。然后将生物素化的蛋白质亲和纯化,并通过质谱分析。我们的分析表明,将常规的蔗糖密度梯度离心法与血浆膜BioID结合使用是克服鉴定完整膜蛋白的固有局限性的理想选择,并且可以产生用于下游蛋白质组学分析的高纯度血浆成分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号