当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimal Dissociation Methods Differ for N- and O-Glycopeptides.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.jproteome.0c00218 Nicholas M Riley 1 , Stacy A Malaker 1 , Marc D Driessen 1 , Carolyn R Bertozzi 1, 2
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.jproteome.0c00218 Nicholas M Riley 1 , Stacy A Malaker 1 , Marc D Driessen 1 , Carolyn R Bertozzi 1, 2
Affiliation
|
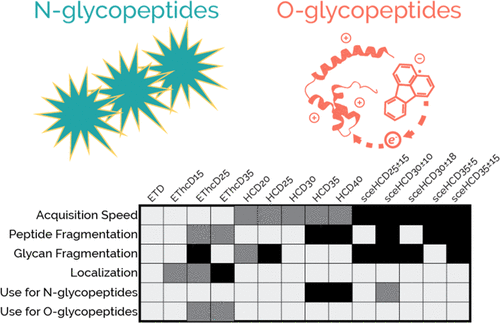
Site-specific characterization of glycosylation requires intact glycopeptide analysis, and recent efforts have focused on how to best interrogate glycopeptides using tandem mass spectrometry (MS/MS). Beam-type collisional activation, i.e., higher-energy collisional dissociation (HCD), has been a valuable approach, but stepped collision energy HCD (sceHCD) and electron transfer dissociation with HCD supplemental activation (EThcD) have emerged as potentially more suitable alternatives. Both sceHCD and EThcD have been used with success in large-scale glycoproteomic experiments, but they each incur some degree of compromise. Most progress has occurred in the area of N-glycoproteomics. There is growing interest in extending this progress to O-glycoproteomics, which necessitates comparisons of method performance for the two classes of glycopeptides. Here, we systematically explore the advantages and disadvantages of conventional HCD, sceHCD, ETD, and EThcD for intact glycopeptide analysis and determine their suitability for both N- and O-glycoproteomic applications. For N-glycopeptides, HCD and sceHCD generate similar numbers of identifications, although sceHCD generally provides higher quality spectra. Both significantly outperform EThcD methods in terms of identifications, indicating that ETD-based methods are not required for routine N-glycoproteomics even if they can generate higher quality spectra. Conversely, ETD-based methods, especially EThcD, are indispensable for site-specific analyses of O-glycopeptides. Our data show that O-glycopeptides cannot be robustly characterized with HCD-centric methods that are sufficient for N-glycopeptides, and glycoproteomic methods aiming to characterize O-glycopeptides must be constructed accordingly.
中文翻译:

N-和O-糖肽的最佳解离方法有所不同。
糖基化的位点特异性表征需要完整的糖肽分析,最近的工作重点是如何使用串联质谱 (MS/MS) 最好地询问糖肽。束型碰撞激活,即高能碰撞解离 (HCD),一直是一种有价值的方法,但阶梯式碰撞能量 HCD (sceHCD) 和电子转移解离与 HCD 补充激活 (EThcD) 已成为可能更合适的替代方案。 sceHCD 和 EThcD 都已成功用于大规模糖蛋白质组实验,但它们各自都会产生一定程度的妥协。大多数进展发生在N-糖蛋白质组学领域。人们越来越有兴趣将这一进展扩展到O-糖蛋白组学,这需要对两类糖肽的方法性能进行比较。在这里,我们系统地探讨了传统 HCD、sceHCD、ETD 和 EThcD 用于完整糖肽分析的优点和缺点,并确定它们对N - 和O - 糖蛋白质组学应用的适用性。对于N-糖肽,HCD 和 sceHCD 生成相似数量的鉴定结果,尽管 sceHCD 通常提供更高质量的光谱。两者在鉴定方面都显着优于 EThcD 方法,这表明常规N-糖蛋白质组学不需要基于 ETD 的方法,即使它们可以生成更高质量的光谱。相反,基于 ETD 的方法,尤其是 EThcD,对于O -糖肽的位点特异性分析是不可或缺的。 我们的数据表明, O-糖肽不能用足以表征N-糖肽的以HCD为中心的方法来稳健地表征,并且必须相应地构建旨在表征O-糖肽的糖蛋白质组学方法。
更新日期:2020-08-08
中文翻译:

N-和O-糖肽的最佳解离方法有所不同。
糖基化的位点特异性表征需要完整的糖肽分析,最近的工作重点是如何使用串联质谱 (MS/MS) 最好地询问糖肽。束型碰撞激活,即高能碰撞解离 (HCD),一直是一种有价值的方法,但阶梯式碰撞能量 HCD (sceHCD) 和电子转移解离与 HCD 补充激活 (EThcD) 已成为可能更合适的替代方案。 sceHCD 和 EThcD 都已成功用于大规模糖蛋白质组实验,但它们各自都会产生一定程度的妥协。大多数进展发生在N-糖蛋白质组学领域。人们越来越有兴趣将这一进展扩展到O-糖蛋白组学,这需要对两类糖肽的方法性能进行比较。在这里,我们系统地探讨了传统 HCD、sceHCD、ETD 和 EThcD 用于完整糖肽分析的优点和缺点,并确定它们对N - 和O - 糖蛋白质组学应用的适用性。对于N-糖肽,HCD 和 sceHCD 生成相似数量的鉴定结果,尽管 sceHCD 通常提供更高质量的光谱。两者在鉴定方面都显着优于 EThcD 方法,这表明常规N-糖蛋白质组学不需要基于 ETD 的方法,即使它们可以生成更高质量的光谱。相反,基于 ETD 的方法,尤其是 EThcD,对于O -糖肽的位点特异性分析是不可或缺的。 我们的数据表明, O-糖肽不能用足以表征N-糖肽的以HCD为中心的方法来稳健地表征,并且必须相应地构建旨在表征O-糖肽的糖蛋白质组学方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号