当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Capturing the Flexibility of a Protein-Ligand Complex: Binding Free Energies from Different Enhanced Sampling Techniques.
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.jctc.9b01150 Sebastian Wingbermühle 1 , Lars V Schäfer 1
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.jctc.9b01150 Sebastian Wingbermühle 1 , Lars V Schäfer 1
Affiliation
|
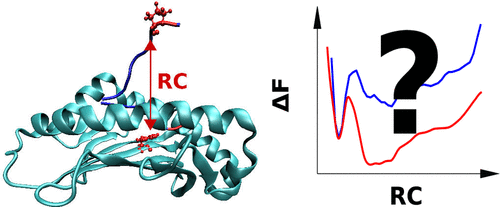
Enhanced sampling techniques are a promising approach to obtain reliable binding free-energy profiles for flexible protein–ligand complexes from molecular dynamics (MD) simulations. To put four popular enhanced sampling techniques to a biologically relevant and challenging test, we studied the partial dissociation of an antigenic peptide from the Major Histocompatibility Complex I (MHC I) HLA-B*35:01 to systematically investigate the performance of umbrella sampling (US), replica exchange with solute tempering 2 (REST2), bias exchange umbrella sampling (BEUS, or replica-exchange umbrella sampling), and well-tempered metadynamics (MTD). With regard to the speed of sampling and convergence, the peptide-MHC I complex (pMHC I) under study showcases intrinsic strengths and weaknesses of the four enhanced sampling techniques used. We found that BEUS can best handle the sampling challenges that arise from the coexistence of an enthalpically and an entropically stabilized free-energy minimum in the pMHC I under study. These findings might also be relevant for other flexible biomolecular systems with competing enthalpically and entropically stabilized minima.
中文翻译:

捕获蛋白质-配体复合物的灵活性:来自不同增强采样技术的结合自由能。
增强的采样技术是一种有前途的方法,可以通过分子动力学(MD)模拟获得可靠的柔性蛋白-配体复合物的结合自由能谱。为了将四种流行的增强采样技术应用于生物学相关且具有挑战性的测试,我们研究了主要组织相容性复合体I(MHC I)HLA-B * 35:01中抗原肽的部分解离,以系统地研究伞状采样的性能(美国),具有溶质回火2(REST2)的副本交换,偏置交换伞式抽样(BEUS或副本交换伞式抽样)和良好的元动力学(MTD)。关于采样和收敛的速度,正在研究的肽-MHC I复合物(pMHC I)展示了所使用的四种增强采样技术的内在优势和劣势。我们发现,BEUS可以最好地应对在研究中的pMHC I中由焓和熵稳定的自由能最小值共同存在引起的采样挑战。这些发现可能与其他在焓和熵稳定的极小值之间竞争的柔性生物分子系统有关。
更新日期:2020-07-14
中文翻译:

捕获蛋白质-配体复合物的灵活性:来自不同增强采样技术的结合自由能。
增强的采样技术是一种有前途的方法,可以通过分子动力学(MD)模拟获得可靠的柔性蛋白-配体复合物的结合自由能谱。为了将四种流行的增强采样技术应用于生物学相关且具有挑战性的测试,我们研究了主要组织相容性复合体I(MHC I)HLA-B * 35:01中抗原肽的部分解离,以系统地研究伞状采样的性能(美国),具有溶质回火2(REST2)的副本交换,偏置交换伞式抽样(BEUS或副本交换伞式抽样)和良好的元动力学(MTD)。关于采样和收敛的速度,正在研究的肽-MHC I复合物(pMHC I)展示了所使用的四种增强采样技术的内在优势和劣势。我们发现,BEUS可以最好地应对在研究中的pMHC I中由焓和熵稳定的自由能最小值共同存在引起的采样挑战。这些发现可能与其他在焓和熵稳定的极小值之间竞争的柔性生物分子系统有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号