当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disruption of the CD Loop by Enzymatic Cleavage Promotes the Formation of Toxic Transthyretin Oligomers through a Common Transthyretin Misfolding Pathway.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.biochem.0c00079 Anvesh K R Dasari 1 , Jenette Arreola 1 , Brian Michael 2 , Robert G Griffin 2 , Jeffery W Kelly 3 , Kwang Hun Lim 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.biochem.0c00079 Anvesh K R Dasari 1 , Jenette Arreola 1 , Brian Michael 2 , Robert G Griffin 2 , Jeffery W Kelly 3 , Kwang Hun Lim 1
Affiliation
|
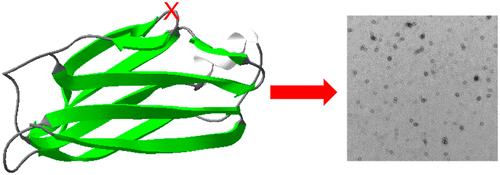
Amyloid formation of full-length TTR involves dissociation of the native tetramers into misfolded monomers that self-assemble into amyloid. In addition to the full-length TTR, C-terminal fragments including residues 49–127 were also observed in vivo, implying the presence of additional misfolding pathways. It was previously proposed that a proteolytic cleavage might lead to the formation of the C-terminal fragment TTR amyloid. Here, we report mechanistic studies of misfolding and aggregation of a TTR variant (G53A) in the absence and presence of a serine protease. A proteolytic cleavage of G53A in the CD loop (K48 and T49) with agitation promoted TTR misfolding and aggregation, suggesting that the proteolytic cleavage may lead to the aggregation of the C-terminal fragment (residues 49–127). To gain more detailed insights into TTR misfolding promoted by proteolytic cleavage, we investigated structural changes in G53A TTR in the presence and absence of trypsin. Our combined biophysical analyses revealed that the proteolytic cleavage accelerated the formation of spherical small oligomers, which exhibited cytotoxic activities. However, the truncated TTR appeared to maintain native-like structures, rather than the C-terminal fragment (residues 49–127) being released and unfolded from the native state. In addition, our solid-state nuclear magnetic resonance and Fourier transform infrared structural studies showed that the two aggregates derived from the full-length and cleaved TTR exhibited nearly identical molecular structural features, suggesting that the proteolytic cleavage in the CD loop destabilizes the native tetrameric structure and accelerates oligomer formation through a common TTR misfolding and aggregation mechanism rather than through a distinct molecular mechanism.
中文翻译:

通过酶促切割破坏 CD 环通过常见的转甲状腺素蛋白错误折叠途径促进有毒转甲状腺素蛋白寡聚体的形成。
全长 TTR 的淀粉样蛋白形成涉及将天然四聚体解离成错误折叠的单体,这些单体自组装成淀粉样蛋白。除了全长 TTR,在体内也观察到包括残基 49-127 的 C 末端片段,意味着存在额外的错误折叠途径。先前提出蛋白水解切割可能导致形成 C 末端片段 TTR 淀粉样蛋白。在这里,我们报告了在不存在和存在丝氨酸蛋白酶的情况下 TTR 变体 (G53A) 的错误折叠和聚集的机制研究。CD 环(K48 和 T49)中 G53A 的蛋白水解切割伴随搅动促进了 TTR 错误折叠和聚集,表明蛋白水解切割可能导致 C 末端片段的聚集(残基 49-127)。为了更详细地了解蛋白水解切割促进的 TTR 错误折叠,我们研究了在胰蛋白酶存在和不存在的情况下 G53A TTR 的结构变化。我们的综合生物物理分析表明,蛋白水解切割加速了球形小低聚物的形成,表现出细胞毒活性。然而,截断的 TTR 似乎保持了类似天然的结构,而不是从天然状态释放和展开的 C 末端片段(残基 49-127)。此外,我们的固态核磁共振和傅里叶变换红外结构研究表明,源自全长和切割 TTR 的两种聚集体表现出几乎相同的分子结构特征,表明 CD 环中的蛋白水解切割使天然四聚体不稳定结构并通过常见的 TTR 错误折叠和聚集机制而不是通过独特的分子机制加速寡聚体的形成。而不是从天然状态释放和展开的 C 末端片段(残基 49-127)。此外,我们的固态核磁共振和傅里叶变换红外结构研究表明,源自全长和切割 TTR 的两种聚集体表现出几乎相同的分子结构特征,表明 CD 环中的蛋白水解切割使天然四聚体不稳定结构并通过常见的 TTR 错误折叠和聚集机制而不是通过独特的分子机制加速寡聚体的形成。而不是从天然状态释放和展开的 C 末端片段(残基 49-127)。此外,我们的固态核磁共振和傅里叶变换红外结构研究表明,源自全长和切割 TTR 的两种聚集体表现出几乎相同的分子结构特征,表明 CD 环中的蛋白水解切割使天然四聚体不稳定结构并通过常见的 TTR 错误折叠和聚集机制而不是通过独特的分子机制加速寡聚体的形成。
更新日期:2020-06-30
中文翻译:

通过酶促切割破坏 CD 环通过常见的转甲状腺素蛋白错误折叠途径促进有毒转甲状腺素蛋白寡聚体的形成。
全长 TTR 的淀粉样蛋白形成涉及将天然四聚体解离成错误折叠的单体,这些单体自组装成淀粉样蛋白。除了全长 TTR,在体内也观察到包括残基 49-127 的 C 末端片段,意味着存在额外的错误折叠途径。先前提出蛋白水解切割可能导致形成 C 末端片段 TTR 淀粉样蛋白。在这里,我们报告了在不存在和存在丝氨酸蛋白酶的情况下 TTR 变体 (G53A) 的错误折叠和聚集的机制研究。CD 环(K48 和 T49)中 G53A 的蛋白水解切割伴随搅动促进了 TTR 错误折叠和聚集,表明蛋白水解切割可能导致 C 末端片段的聚集(残基 49-127)。为了更详细地了解蛋白水解切割促进的 TTR 错误折叠,我们研究了在胰蛋白酶存在和不存在的情况下 G53A TTR 的结构变化。我们的综合生物物理分析表明,蛋白水解切割加速了球形小低聚物的形成,表现出细胞毒活性。然而,截断的 TTR 似乎保持了类似天然的结构,而不是从天然状态释放和展开的 C 末端片段(残基 49-127)。此外,我们的固态核磁共振和傅里叶变换红外结构研究表明,源自全长和切割 TTR 的两种聚集体表现出几乎相同的分子结构特征,表明 CD 环中的蛋白水解切割使天然四聚体不稳定结构并通过常见的 TTR 错误折叠和聚集机制而不是通过独特的分子机制加速寡聚体的形成。而不是从天然状态释放和展开的 C 末端片段(残基 49-127)。此外,我们的固态核磁共振和傅里叶变换红外结构研究表明,源自全长和切割 TTR 的两种聚集体表现出几乎相同的分子结构特征,表明 CD 环中的蛋白水解切割使天然四聚体不稳定结构并通过常见的 TTR 错误折叠和聚集机制而不是通过独特的分子机制加速寡聚体的形成。而不是从天然状态释放和展开的 C 末端片段(残基 49-127)。此外,我们的固态核磁共振和傅里叶变换红外结构研究表明,源自全长和切割 TTR 的两种聚集体表现出几乎相同的分子结构特征,表明 CD 环中的蛋白水解切割使天然四聚体不稳定结构并通过常见的 TTR 错误折叠和聚集机制而不是通过独特的分子机制加速寡聚体的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号